Ijcep0076955.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Prevalence and Incidence of Rare Diseases: Bibliographic Data
Number 1 | January 2019 Prevalence and incidence of rare diseases: Bibliographic data Prevalence, incidence or number of published cases listed by diseases (in alphabetical order) www.orpha.net www.orphadata.org If a range of national data is available, the average is Methodology calculated to estimate the worldwide or European prevalence or incidence. When a range of data sources is available, the most Orphanet carries out a systematic survey of literature in recent data source that meets a certain number of quality order to estimate the prevalence and incidence of rare criteria is favoured (registries, meta-analyses, diseases. This study aims to collect new data regarding population-based studies, large cohorts studies). point prevalence, birth prevalence and incidence, and to update already published data according to new For congenital diseases, the prevalence is estimated, so scientific studies or other available data. that: Prevalence = birth prevalence x (patient life This data is presented in the following reports published expectancy/general population life expectancy). biannually: When only incidence data is documented, the prevalence is estimated when possible, so that : • Prevalence, incidence or number of published cases listed by diseases (in alphabetical order); Prevalence = incidence x disease mean duration. • Diseases listed by decreasing prevalence, incidence When neither prevalence nor incidence data is available, or number of published cases; which is the case for very rare diseases, the number of cases or families documented in the medical literature is Data collection provided. A number of different sources are used : Limitations of the study • Registries (RARECARE, EUROCAT, etc) ; The prevalence and incidence data presented in this report are only estimations and cannot be considered to • National/international health institutes and agencies be absolutely correct. -

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -

Mackenzie's Mission Gene & Condition List
Mackenzie’s Mission Gene & Condition List What conditions are being screened for in Mackenzie’s Mission? Genetic carrier screening offered through this research study has been carefully developed. It is focused on providing people with information about their chance of having children with a severe genetic condition occurring in childhood. The screening is designed to provide genetic information that is relevant and useful, and to minimise uncertain and unclear information. How the conditions and genes are selected The Mackenzie’s Mission reproductive genetic carrier screen currently includes approximately 1300 genes which are associated with about 750 conditions. The reason there are fewer conditions than genes is that some genetic conditions can be caused by changes in more than one gene. The gene list is reviewed regularly. To select the conditions and genes to be screened, a committee comprised of experts in genetics and screening was established including: clinical geneticists, genetic scientists, a genetic pathologist, genetic counsellors, an ethicist and a parent of a child with a genetic condition. The following criteria were developed and are used to select the genes to be included: • Screening the gene is technically possible using currently available technology • The gene is known to cause a genetic condition • The condition affects people in childhood • The condition has a serious impact on a person’s quality of life and/or is life-limiting o For many of the conditions there is no treatment or the treatment is very burdensome for the child and their family. For some conditions very early diagnosis and treatment can make a difference for the child. -

Tracheobronchial Stenosis in Keutel Syndrome
C O R R E S P O N D E N C E Tracheobronchial Stenosis in Keutel Syndrome Keutel syndrome is characterized by brachytelephalangism, abnormal cartilage calcification, peripheral pulmonary stenoses, and midfacial hypoplasia. We report the first case from East Asia in an 8-month-old boy who had the typical craniofacial appearance characterized by midfacial hypoplasia with a broad depressed nasal bridge (Fig. 1). The distal phalanges of fingers were thickened. Auscultation FIG.1 Midface hypoplasia is present with a depressed nasal revealed a grade 2-3/6 systolic murmur over heart, bridge and small nose. pronounced in the second and third intercostal space, and an inspiratory and expiratory stridor and wheezing over both lungs. Chest radiograph and computed tomography alternative to surgical resection. Endoscopy has been showed tracheobronchial cartilage calcification and suggested as the first choice for simple stenosis, and tracheobronchial stenosis, confirmed on bronchoscopy. success rate of 96% has been reported. So far, this Echocardiography revealed peripheral pulmonary approach has rarely been used in children. Our patient stenosis. accepted bronchoscopic cryotherapy and balloon dilatation four times, and the diameter of the subglottic Keutel syndrome is a rare autosomal recessive laryngeal stenosis was expanded from 3 mm to 4.5 mm. disease, with 27 reported cases from 19 families in The clinical symptoms improved after endoscopy, but he several countries; mostly from the Middle East. All of died of lung reinfection three weeks after discharge from them showed tracheobronchial calcification, and five of our hospital. them had stenosis of the tracheobronchial tree [1,2]. Our LI-FENG SUN AND XING CHEN, patient is the fifth patient with tracheobronchial stenosis, Department of Pediatrics, Provincial Hospital Affiliated to which should be emphasized as another remarkable Shandong University, Jinan, 250021, China. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub
US 2010O2.10567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub. Date: Aug. 19, 2010 (54) USE OF ATUFTSINASATHERAPEUTIC Publication Classification AGENT (51) Int. Cl. A638/07 (2006.01) (76) Inventor: Dorian Bevec, Germering (DE) C07K 5/103 (2006.01) A6IP35/00 (2006.01) Correspondence Address: A6IPL/I6 (2006.01) WINSTEAD PC A6IP3L/20 (2006.01) i. 2O1 US (52) U.S. Cl. ........................................... 514/18: 530/330 9 (US) (57) ABSTRACT (21) Appl. No.: 12/677,311 The present invention is directed to the use of the peptide compound Thr-Lys-Pro-Arg-OH as a therapeutic agent for (22) PCT Filed: Sep. 9, 2008 the prophylaxis and/or treatment of cancer, autoimmune dis eases, fibrotic diseases, inflammatory diseases, neurodegen (86). PCT No.: PCT/EP2008/007470 erative diseases, infectious diseases, lung diseases, heart and vascular diseases and metabolic diseases. Moreover the S371 (c)(1), present invention relates to pharmaceutical compositions (2), (4) Date: Mar. 10, 2010 preferably inform of a lyophilisate or liquid buffersolution or artificial mother milk formulation or mother milk substitute (30) Foreign Application Priority Data containing the peptide Thr-Lys-Pro-Arg-OH optionally together with at least one pharmaceutically acceptable car Sep. 11, 2007 (EP) .................................. O7017754.8 rier, cryoprotectant, lyoprotectant, excipient and/or diluent. US 2010/0210567 A1 Aug. 19, 2010 USE OF ATUFTSNASATHERAPEUTIC ment of Hepatitis BVirus infection, diseases caused by Hepa AGENT titis B Virus infection, acute hepatitis, chronic hepatitis, full minant liver failure, liver cirrhosis, cancer associated with Hepatitis B Virus infection. 0001. The present invention is directed to the use of the Cancer, Tumors, Proliferative Diseases, Malignancies and peptide compound Thr-Lys-Pro-Arg-OH (Tuftsin) as a thera their Metastases peutic agent for the prophylaxis and/or treatment of cancer, 0008. -

Risk Factors for Major Adverse Events After Surgical Closure of Ventricular Septal Defect in Patients Less Than 1 Year of Age: a Single-Center Retrospective
ORIGINAL ARTICLE Braz J Cardiovasc Surg 2019;34(3):335-43 Risk Factors for Major Adverse Events after Surgical Closure of Ventricular Septal Defect in Patients Less than 1 Year of Age: A Single-Center Retrospective Servet Ergün1, MD; Serhat Bahadır Genç1, MD; Okan Yildiz1, MD; Erkut Öztürk2, MD; Hasan Candaş Kafalı2, MD; Pelin Ayyıldız2, MD; Sertaç Haydin1, MD DOI: 10.21470/1678-9741-2018-0299 Abstract Objective: To reveal the risk factors that can lead to a permanent pacemaker implantation. Hemodynamically complicated course and an increased morbidity in patients < 1 significant residual VSD was observed in six (3.2%) patients. year old after surgical ventricular septal defect (VSD) closure. Extracorporeal membrane oxygenation-cardiopulmonary Methods: We reviewed a consecutive series of patients who resuscitation was performed in one (0.5%) patient. Small age (< 4 were admitted to our institution for surgical VSD closure who months) (P-value<0.001) and prolonged cardiopulmonary bypass were under one year of age, between 2015 and 2018. Mechanical time (P=0.03) were found to delay extubation and to prolong ventilation (MV) time > 24 hours, intensive care unit (ICU) stay MV time. Low birth weight at the operation was associated with longer than three days, and hospital stay longer than seven days MAE (P=0.03). were defined as “prolonged”. Unplanned reoperation, complete Conclusion: Higher body weight during operation had a heart block requiring a permanent pacemaker implantation, reducing effect on the MAE frequency and shortened the MV sudden circulatory arrest, and death were considered as duration, ICU stay, and hospital stay. As a conclusion, for patients significant major adverse events (MAE). -

Discover Dysplasias Gene Panel
Discover Dysplasias Gene Panel Discover Dysplasias tests 109 genes associated with skeletal dysplasias. This list is gathered from various sources, is not designed to be comprehensive, and is provided for reference only. This list is not medical advice and should not be used to make any diagnosis. Refer to lab reports in connection with potential diagnoses. Some genes below may also be associated with non-skeletal dysplasia disorders; those non-skeletal dysplasia disorders are not included on this list. Skeletal Disorders Tested Gene Condition(s) Inheritance ACP5 Spondyloenchondrodysplasia with immune dysregulation (SED) AR ADAMTS10 Weill-Marchesani syndrome (WMS) AR AGPS Rhizomelic chondrodysplasia punctata type 3 (RCDP) AR ALPL Hypophosphatasia AD/AR ANKH Craniometaphyseal dysplasia (CMD) AD Mucopolysaccharidosis type VI (MPS VI), also known as Maroteaux-Lamy ARSB syndrome AR ARSE Chondrodysplasia punctata XLR Spondyloepimetaphyseal dysplasia with joint laxity type 1 (SEMDJL1) B3GALT6 Ehlers-Danlos syndrome progeroid type 2 (EDSP2) AR Multiple joint dislocations, short stature and craniofacial dysmorphism with B3GAT3 or without congenital heart defects (JDSCD) AR Spondyloepimetaphyseal dysplasia (SEMD) Thoracic aortic aneurysm and dissection (TADD), with or without additional BGN features, also known as Meester-Loeys syndrome XL Short stature, facial dysmorphism, and skeletal anomalies with or without BMP2 cardiac anomalies AD Acromesomelic dysplasia AR Brachydactyly type A2 AD BMPR1B Brachydactyly type A1 AD Desbuquois dysplasia CANT1 Multiple epiphyseal dysplasia (MED) AR CDC45 Meier-Gorlin syndrome AR This list is gathered from various sources, is not designed to be comprehensive, and is provided for reference only. This list is not medical advice and should not be used to make any diagnosis. -

Downloaded from Here
bioRxiv preprint doi: https://doi.org/10.1101/017566; this version posted November 19, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 Testing for ancient selection using cross-population allele 2 frequency differentiation 1;∗ 3 Fernando Racimo 4 1 Department of Integrative Biology, University of California, Berkeley, CA, USA 5 ∗ E-mail: [email protected] 6 1 Abstract 7 A powerful way to detect selection in a population is by modeling local allele frequency changes in a 8 particular region of the genome under scenarios of selection and neutrality, and finding which model is 9 most compatible with the data. Chen et al. [2010] developed a composite likelihood method called XP- 10 CLR that uses an outgroup population to detect departures from neutrality which could be compatible 11 with hard or soft sweeps, at linked sites near a beneficial allele. However, this method is most sensitive 12 to recent selection and may miss selective events that happened a long time ago. To overcome this, 13 we developed an extension of XP-CLR that jointly models the behavior of a selected allele in a three- 14 population tree. Our method - called 3P-CLR - outperforms XP-CLR when testing for selection that 15 occurred before two populations split from each other, and can distinguish between those events and 16 events that occurred specifically in each of the populations after the split. -
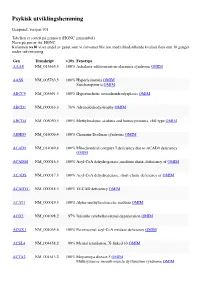
Psykisk Utviklingshemming
Psykisk utviklingshemming Genpanel, versjon v01 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC Kolonnen >x10 viser andel av genet som vi forventer blir lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering Gen Transkript >10x Fenotype AAAS NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AASS NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCC9 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 NM_000033.3 76% Adrenoleukodystrophy OMIM ABCD4 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 NM_014049.4 100% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL NM_000018.3 100% VLCAD deficiency OMIM ACAT1 NM_000019.3 100% Alpha-methylacetoacetic aciduria OMIM ACO2 NM_001098.2 97% Infantile cerebellar-retinal degeneration OMIM ACOX1 NM_004035.6 100% Peroxisomal acyl-CoA oxidase deficiency OMIM ACSL4 NM_004458.2 99% Mental retardation, X-linked 63 OMIM ACTA2 NM_001613.2 100% Moyamoya disease 5 OMIM Multisystemic smooth muscle dysfunction syndrome OMIM Gen Transkript >10x Fenotype ACTB NM_001101.3 100% ?Dystonia, juvenile-onset OMIM Baraitser-Winter syndrome 1 OMIM ACTG1 NM_001614.3 100% Baraitser-Winter syndrome 2 OMIM Deafness, autosomal dominant 20/26 OMIM ACVR1 NM_001105.4 100% Fibrodysplasia ossificans -

Identification of Shared and Unique Gene Families Associated with Oral
International Journal of Oral Science (2017) 9, 104–109 OPEN www.nature.com/ijos ORIGINAL ARTICLE Identification of shared and unique gene families associated with oral clefts Noriko Funato and Masataka Nakamura Oral clefts, the most frequent congenital birth defects in humans, are multifactorial disorders caused by genetic and environmental factors. Epidemiological studies point to different etiologies underlying the oral cleft phenotypes, cleft lip (CL), CL and/or palate (CL/P) and cleft palate (CP). More than 350 genes have syndromic and/or nonsyndromic oral cleft associations in humans. Although genes related to genetic disorders associated with oral cleft phenotypes are known, a gap between detecting these associations and interpretation of their biological importance has remained. Here, using a gene ontology analysis approach, we grouped these candidate genes on the basis of different functional categories to gain insight into the genetic etiology of oral clefts. We identified different genetic profiles and found correlations between the functions of gene products and oral cleft phenotypes. Our results indicate inherent differences in the genetic etiologies that underlie oral cleft phenotypes and support epidemiological evidence that genes associated with CL/P are both developmentally and genetically different from CP only, incomplete CP, and submucous CP. The epidemiological differences among cleft phenotypes may reflect differences in the underlying genetic causes. Understanding the different causative etiologies of oral clefts is -

STAMBP Gene STAM Binding Protein
STAMBP gene STAM binding protein Normal Function The STAMBP gene provides instructions for making a protein called STAM binding protein. Although its exact function is not well understood, within cells this protein interacts with large groups of interrelated proteins known as endosomal sorting complexes required for transport (ESCRTs). ESCRTs help transport proteins from the outer cell membrane to the interior of the cell, a process known as endocytosis. In particular, they are involved in the endocytosis of damaged or unneeded proteins that need to be broken down (degraded) or recycled by the cell. ESCRTs help sort these proteins into structures called multivesicular bodies (MVBs), which deliver them to lysosomes. Lysosomes are compartments within cells that digest and recycle many different types of molecules. Through its association with ESCRTs, STAM binding protein helps to maintain the proper balance of protein production and breakdown (protein homeostasis) that cells need to function and survive. Studies suggest that the interaction of STAM binding protein with ESCRTs is also involved in multiple chemical signaling pathways within cells, including pathways needed for overall growth and the formation of new blood vessels (angiogenesis). Health Conditions Related to Genetic Changes Microcephaly-capillary malformation syndrome At least 13 mutations in the STAMBP gene have been identified in people with microcephaly-capillary malformation syndrome, an inherited disorder characterized by an abnormally small head size (microcephaly), profound developmental delay and intellectual disability, recurrent seizures (epilepsy), and abnormalities of small blood vessels in the skin called capillaries (capillary malformations). The known STAMBP gene mutations reduce or eliminate the production of STAM binding protein.