Expert Report of Murray Wilson
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intellectual Property Outlook: Cases and Trends to Follow in 2012
February 2012 Intellectual Property Outlook: Cases and Trends to Follow in 2012 BY ROBERT M. MASTERS, TAD RICHMAN, & LISA LEUNG I. Introduction In the coming year, we anticipate a series of events and decisions with varying degrees of impact on intellectual property law in the United States and, in turn, the way we advise our clients. This article highlights cases we are monitoring that present, in our view, significant issues relating to various aspects of intellectual property law. Most of these cases are pending appeal at some level, and each has the potential for considerable impact on the landscape of U.S. intellectual property law. A small number of recently decided cases have also been selected for inclusion in this discussion, as it remains to be seen precisely how these decisions will play out in practice over the coming year. Lastly, we take a look at continuing trends in the intellectual property marketplace and review key legislation, including what to expect from the recently enacted America Invents Act. II. Cases to Watch in 2012 A. The Scope of Patentable Subject Matter 1. Medical Diagnostic and Treatment Methods Post-Bilski The Supreme Court heard oral argument in Mayo Collaborative Svcs. v. Prometheus Labs., Inc., No. 10-1150, on December 7, 2011. This is not the first time the Supreme Court has taken on the question of the validity of Prometheus’s asserted method claims. As before, Mayo challenges the Federal Circuit’s holding that Prometheus’s method claims recite patentable subject matter under §101. Unlike before, Mayo’s appeal now derives from a post-Bilski decision below.1 Prometheus is the sole and exclusive licensee of two patents claiming methods for determining the optimal dosage of thiopurine drugs used to treat certain autoimmune diseases. -

Of Japanese Patent Prosecution
The ‘Endless Loop’ of Japanese Patent Prosecution By Samson Helfgott and Paula E. Hopkins Katten Muchin Rosenman LLP First published in the May 2006 issue of World Intellectual Property Report Commentary The ‘Endless Loop’ of Japanese Patent Prosecution By Samson Helfgott and Paula E.Hopkins.Samson Helfgott During the course of the appeal trial, the Trial Examiners are is a Partner and Director of Patents, and Paula E. Hopkins is allowed to find a new reason and/or new prior art to reject the an associate in the IP Department of Katten Muchin patent application other than those stipulated in the decision of the Rosenman LLP, New York. The authors can be contacted examiner (Section 150, subsection 1 and Section 153, subsection by e-mail at: [email protected] and 1). In such case, the Board has to notify the new reason and/or [email protected] prior art to the applicant and allow the applicant to make counter-arguments against that reason and/or prior art before Patent prosecution in any patent system has its normal course of issuance of the decision of the Board (Section 159, subsection 2 delays. In significant cases, especially when broad claims are and Section 50). Accordingly, by way of example, although the being prosecuted, even more time may be required until a examiner may have only rejected the claim based upon certain resolution of the patent issues is reached. However, within the sections, such as lack of novelty, the Board of Trial Examiners can Japanese Patent System peculiarities within the law make such reject those claims for other reasons, such as indefiniteness, lack delays indefinite, resulting in an “endless loop” of prosecution of support, etc. -

Patenting: a Guidebook for Patenting in a Post-America Invents Act World
Foley Hoag eBook PATENTING: A Guidebook For Patenting in a Post-America Invents Act World by Beth E. Arnold PATENTING: A GUIDEBOOK FOR PATENTING IN A POST-AMERICA INVENTS ACT WORLD IN A POST-AMERICA A GUIDEBOOK FOR PATENTING PATENTING: 1 Contents Preface ............................................................................................................1 Chapter 1 What Is a Patent? ..........................................................................................2 Chapter 2 What Is Potentially Patentable? ................................................................5 Chapter 3 What Is Not Patentable? .............................................................................7 Chapter 4 How Is a Patent Obtained? .......................................................................16 Chapter 5 What Should You Do Before Filing a Patent Application? ...............25 Chapter 6 What Shouldn’t You Do Before Filing a Patent Application? ...........28 Chapter 7 How Are Foreign Patents Obtained? .....................................................29 Chapter 8 Who Is an Inventor on a Patent? ............................................................33 Chapter 9 Who Owns the Patent? ..............................................................................35 Chapter 10 How Long Is a Patent in Effect? .............................................................39 About Foley Hoag ...............................................................................................42 About the Author: Beth E. Arnold .........................................................................43 -

Patent Prosecution from an Examiner's Perspective
January 31, 2019 31, January Gray Andrew and Pezzner Benjamin ALLOWANCE THE ATTAINING AND ACTION OFFICE THE ANTICIPATING EXAMINER’SFROM AN PERSPECTIVE: PROSECUTION PATENT © 2019 Morgan, Lewis & Bockius LLP Presenter Background • Patent Examiner: 3 years – 3 different art units; my last art unit was run very well, we followed all of the rules, primaries were very good, SPE was fair, I didn't understand the criticism from the blogs – goal was to document the thinking of an examiner so I could use it on the outside • Patent Attorney: 2 years – expected the goal to be outwitting examiners and getting allowances – goal turned out to be attaining higher quality examination (with allowances being a byproduct) 2 Presentation Goal • Make Examination Great Again! – show you how to get patent examiners to follow their own rules, to follow their training, to be more accountable – more accountability = higher quality examination = more allowances 3 Agenda 1. Day in the life of an examiner – motivations, training, oversight 2. Using rules to get leverage – the rules that, if broken, result in a do-over 3. Using leverage to move prosecution forward – without losing examiner goodwill (diplomatic vs. adversarial); interviews 4. When diplomacy fails – steps to take before appeal 5. 101 developments – examiner training, thoughts on implementation 4 PART 1 DAY IN THE LIFE OF AN EXAMINER Day in the Life: Motivation • Evaluated based on quantity (production) and quality (master review form) • Main motivation: work as quickly and efficiently as possible (quantity) -

Patenting Fast and Slow: Examiner and Applicant Use of Prior Art
Law Faculty Scholarship WVU College of Law 2020 Patenting Fast and Slow: Examiner and Applicant Use of Prior Art Shine Tu Follow this and additional works at: https://researchrepository.wvu.edu/law_faculty Part of the Intellectual Property Law Commons PATENTING FAST AND SLOW: EXAMINER AND APPLICANT USE OF PRIOR ART* SHINE SEAN Tu* ABSTRACT Previous studies have shown that an applicant'sability to obtain a patent is inexorably linked to the random assignment of a patent examiner.1 However, not all patent examiners are created equal. Some patent examiners allow patent applicationsquickly within just one or two Office Actions, resulting in only a few months of substantive patent prosecution. In contrast, otherpatent examiners constantly rejectpatents applications, which can result in unnecessarilydelaying prosecution and years of substantive patent prosecution. This study focuses on how different examiners use prior art rejections to prolong or compact prosecution. Prior art rejections are one of the most important hurdles to obtaining a patent. Specifically, the use of prior art rejections directly impacts the time and effort it takes to obtain a patent. Anticipation and obviousness rejections are usually the most important and difficult *Permission is hereby granted for noncommercial reproduction of this Article in whole or in part for education or research purposes, including the making of multiple copies for classroom use, subject only to the condition that the name of the author, a complete citation, and this copyright notice and grant -

Collusion and Collective Action in the Patent System: a Proposal for Patent Bounties
Georgetown University Law Center Scholarship @ GEORGETOWN LAW 2001 Collusion and Collective Action in the Patent System: A Proposal for Patent Bounties John R. Thomas Georgetown University Law Center, [email protected] This paper can be downloaded free of charge from: https://scholarship.law.georgetown.edu/facpub/306 2001 U. Ill. L. Rev. 305-353 (2001) This open-access article is brought to you by the Georgetown Law Library. Posted with permission of the author. Follow this and additional works at: https://scholarship.law.georgetown.edu/facpub Part of the Courts Commons, and the Intellectual Property Law Commons GEORGETOWN LAW Faculty Publications March 2010 Collusion and Collective Action in the Patent System: A Proposal for Patent Bounties 2001 U. Ill. L. Rev. 305-353 (2001) John R. Thomas Professor of Law Georgetown University Law Center [email protected] This paper can be downloaded without charge from: Scholarly Commons: http://scholarship.law.georgetown.edu/facpub/306/ Posted with permission of the author COLLUSION AND COLLECTIVE ACTION IN THE PATENT SYSTEM: A PROPOSAL FOR PATENT BOUNTIES John R. Thomas* Persistent commentary contends that the Patent Office is issuing patents that appropriate public domain concepts at an alarming fre quency. Complaints of low patent quality enjoy growing resonance with regard to business methods, computer software, and other inven tions for which patents were not traditionally sought. In this article, Professor Jay Thomas explains how the judiciary's lenient view of patentable subject matter and utility standards, along with miserly congressional funding policies, have rendered the Patent Office an in creasingly porous agency. -

Amazon Jungle K the “One-Click” Patent and Its Reexamination
early three Ndecades ago the U.S. Supreme Court, in Diamond v. Diehr K CASE COMMENT Claim 11 states: art. Nevertheless, he stated that claims 1 and reexamination helps the USPTO to A method for ordering an item using a 11 would be considered patentable if they compensate for the shortcomings of a purely client system, the method comprising: were amended to include a shopping cart ex parte examination process. displaying information identifying the model as an element of the claim. The history of the Amazon.com One-Click item and displaying an indication of a On November 29, 2007, Amazon.com patent reminds one of the authors of this single action that is to be performed to responded to the Office Action with proposed comment of his own experiences in dealing with order the identified item; and in response to amendments to claims 1 and 11. Amazon.com’s another controversial software patent, U.S. only the indicated single action being proposed amendment to claim 11 reads: Patent No. 5,241,671, issued to Compton’s performed, sending to a server system a A method for ordering an item using a Multimedia early in the digital age in 1993. request to order the identified item whereby client system, the method comprising: Shortly after taking office as Commissioner of the item is ordered independently of a displaying information identifying the the USPTO he faced a public furor over the shopping cart model and the order is item purchasable through a shopping cart issuance of this patent, which covered a wide fulfilled to complete a purchase of the item. -

Office Actions, Notices of Allowance, and Other Office Notices Previously Mailed to Areas of Texas and Louisiana Affected by Hurricane Harvey
Office Actions, Notices of Allowance, and Other Office Notices Previously Mailed to Areas of Texas and Louisiana Affected by Hurricane Harvey The United States Patent and Trademark Office (USPTO) has issued a notice announcing that the USPTO considers the effects of Hurricane Harvey in Texas and Louisiana that began on August 25, 2017, to be an "extraordinary situation" within the meaning of37 CPR 1.183. That notice provides additional relief for affected applicants and reexamination parties in certain situations. See https://www.uspto.gov/learning-and-resources/operational-status/customers-affected-hurricane-harvey. The instant notice supplements that notice. For patent applications and reexamination proceedings pending in the USPTO as of August 25, 2017, having a correspondence address in the areas of Texas and Louisiana affected by Hurricane Harvey that the USPTO has identified as being in affected areas based on the zip code ofthe correspondence address, in which a reply to an Office action (final, non-final, or other), a notice of allowance, or other Office notice (hereinafter collectively referred to as "Office communication") is outstanding, and for which the statutory or non-statutory time period set for reply has not yet expired, the USPTO is withdrawing the Office communication, and re-issuing the Office communication with a new time period for reply. See MPEP § 710.06 (Situations When Reply Period Is Reset or Restarted). The Office communication must have been outstanding on August 25, 2017. Note that the USPTO will not restart any time period for reply once the statutory or non-statutory time period has expired. The United States Postal Service (USPS) has indicated that there is a service disruption in the areas of Texas and Louisiana affected by Hurricane Harvey. -
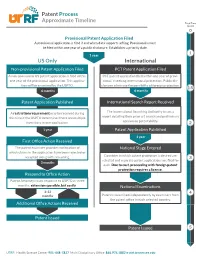
Patent Process Approximate Timeline US Only International
Patent Process Approximate Timeline Total Time (years) Provisional Patent Application Filed A provisional application is filed if and when data supports a filing. Provisionals must be filed within one year of a public disclosure. Establishes a priority date. 1 year US Only International Non-provisional Patent Application Filed PCT Patent Application Filed A non-provisional US patent application is filed within PCT patent application filed within one year of provi- one year of the provisional application. This applica- sional, if seeking international protection. Public dis- tion will be examined by the USPTO. closures eliminate the possibility of foreign protection. 6 months 6 months Patent Application Published International Search Report Received A restriction requirement may be received during The International Searching Authority issues a this time if the USPTO determines there are multiple report detailing their prior art search and preliminary inventions in one application. opinion on patentability. 1 year Patent Application Published 1 year First Office Action Received The patent examiner provides notification of National Stage Entered which claims in the application have been rejected or accepted along with reasoning. Countries in which patent protection is desired are selected and separate patent applications are filed for 3 months each. Due to cost, proceeding with foreign patent protection requires a license. Respond to Office Action Patent Attorney issues response to USPTO in three months, extensions possible, but costly. National Examinations 3-12 months Patent is examined independently by examiners from the patent office in each selected country. Additional Office Actions Received Patent Issued Patent Issued UTRF: Health Science Center 901-448-7827 Multi Disciplinary Office 865.974.1882 • utrf.tennessee.edu. -

Final Office Action Rejections for Patent Applications: It Ain’T Over Until the Fat Lady Sings…Usually John W
PROdUcT ProtecTiOn And ThE LAw NEWS Final Office Action Rejections for Patent Applications: It Ain’t Over Until the Fat Lady Sings…Usually John W. Boger, Esq. Heslin Rothenberg Farley & Mesiti P.C. The patent process can be a long and, at times, tedious process. specification, claims or drawings, new arguments or new evidence Many inventors assume that once you file your application for supporting patentability), the examiner will withdraw the with the United States Patent and Trademark Office (USPTO), finality of the Office Action and the submission will be entered the hard work is done. Unfortunately, for most cases, the and considered by the examiner. Essentially, the RCE allows hard work has just begun. It is highly unusual for a patent the inventor to file a second application to keep the prosecution application to not be rejected and receive at least one Office of the first application alive. The RCE application stays with Action from the patent examiner. Typically, inventors will the same examiner that was reviewing the first application receive multiple Office Actions in which their invention is and keeps the same application serial number. Filing an RCE is rejected for any number of reasons, including lack of novelty, capitalism at its best: pay more money and file a submission to obviousness and ineligible subject matter. When an inventor buy another full examination of your application. However, a receives a Final Office Action, he has now come to a critical potential downside to filing an RCE is, since the application is crossroads in the prosecution highway. The inventor may staying with the same examiner, that examiner may just repeat choose from three options: (1) abandon the application, (2) file the same basis for his rejections without considering the newly a Request for Continued Examination and pay a fee or (3) file filed arguments. -

Snell&Wilmer
Snell & Wilmer Understanding what makes you unique.® Ten Tips on Preparing and Prosecuting Patent Applications August 17, 2017 by David E. Rogers 1. Keep Your Eyes on the Prize. A patent defines a piece of intellectual property. As with a parcel of land, the patent’s owner can legally prevent any trespass (called an infringement) on the patent. Analogous to the size of a parcel of land, the size of a patent (usually referred to as its scope) is critical in determining its value. Mistakes can mean the difference between a patent worth millions and a worthless piece of paper. Patents prepared and/or prosecuted with little forethought about the end game, which is commercializing, selling or licensing the invention, often have little value. Mistakes can mean the difference between a patent worth millions and a worthless piece of paper. 2. Obtaining a Patent Is Not the Goal; Obtaining Broad Claim Scope Is the Goal. (a) Merely obtaining a patent is not the goal. The goal is to maximize the scope of meaningful patent protection to which your invention is entitled. Put yourself in a competitor’s shoes - how would you design around the claims in your patent application? If you can conceive a practical design-around option, modify the claims and plug the gap if possible. By initially preparing narrow claims, or unnecessarily narrowing claims during prosecution, you create design-around opportunities competitors can use to circumvent the patent. (b) To support broad claim scope, the written description of the invention and drawings should be detailed and thorough. (c) Patent prosecution is not a “negotiation” with a USPTO Examiner, unless there is a misunderstanding or minor disagreement. -

An Empirical Study on the Effects of the America Invents Act on Patent Applications Owned by Small Businesses
An Empirical Study on the Effects of the America Invents Act on Patent Applications Owned by Small Businesses Yoo Jeong Han Department of Statistics University of California, Los Angeles February 2021 Abstract This paper evaluates the heterogenous impacts of the America Invents Act of 2011 (AIA) on patent applications for small and large businesses. Using data collected from the United States Patent and Trademark Office and Google Patents, I compare how the probability of suc- cessfully overcoming an initial rejection is affected by the AIA for small- and large-business applicants, respectively. This comparison is achieved by analyzing the data using a difference- in-differences approach. Results suggest that after the enactment of the AIA, small-business applicants were relatively favored when compared against large-business applicants. This ef- fect is statistically significant and also practically large. arXiv:2102.02160v1 [stat.AP] 3 Feb 2021 1 1 Introduction It has been argued that small businesses have been unfavorably affected by the major reforms (Braun, 2012) of the Leahy-Smith America Invents Act of 2011 (AIA). Vandenburg (2014) and Sutton (2014) propose that the transition from the First-To-Invent system to the First-To-File sys- tem (Section 3, AIA) favors large businesses that have the financial resources to accommodate faster filing, thus placing small businesses at a relative disadvantage. Case (2013) notes the AIA’s elimination of the post-disclosure grace period as another source of disadvantage. The lack of a post-disclosure grace period forces inventors to either file before fully developing their ideas or to fully develop their ideas while at risk of premature disclosure by others.