Pathogenic Variants in the DEAH-Box RNA Helicase DHX37 Are a Frequent Cause of 46,XY Gonadal Dysgenesis and 46,XY Testicular Regression Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Background Briefing Document from the Consortium of Sponsors for The
BRIEFING BOOK FOR Pediatric Advisory Committee (PAC) Prepared by Alan Rogol, M.D., Ph.D. Prepared for Consortium of Sponsors Meeting Date: April 8th, 2019 TABLE OF CONTENTS LIST OF FIGURES ................................................... ERROR! BOOKMARK NOT DEFINED. LIST OF TABLES ...........................................................................................................................4 1. INTRODUCTION AND BACKGROUND FOR THE MEETING .............................6 1.1. INDICATION AND USAGE .......................................................................................6 2. SPONSOR CONSORTIUM PARTICIPANTS ............................................................6 2.1. TIMELINE FOR SPONSOR ENGAGEMENT FOR PEDIATRIC ADVISORY COMMITTEE (PAC): ............................................................................6 3. BACKGROUND AND RATIONALE .........................................................................7 3.1. INTRODUCTION ........................................................................................................7 3.2. PHYSICAL CHANGES OF PUBERTY ......................................................................7 3.2.1. Boys ..............................................................................................................................7 3.2.2. Growth and Pubertal Development ..............................................................................8 3.3. AGE AT ONSET OF PUBERTY.................................................................................9 3.4. -

Analysis of Gene Expression Data for Gene Ontology
ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Master of Science Robert Daniel Macholan May 2011 ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION Robert Daniel Macholan Thesis Approved: Accepted: _______________________________ _______________________________ Advisor Department Chair Dr. Zhong-Hui Duan Dr. Chien-Chung Chan _______________________________ _______________________________ Committee Member Dean of the College Dr. Chien-Chung Chan Dr. Chand K. Midha _______________________________ _______________________________ Committee Member Dean of the Graduate School Dr. Yingcai Xiao Dr. George R. Newkome _______________________________ Date ii ABSTRACT A tremendous increase in genomic data has encouraged biologists to turn to bioinformatics in order to assist in its interpretation and processing. One of the present challenges that need to be overcome in order to understand this data more completely is the development of a reliable method to accurately predict the function of a protein from its genomic information. This study focuses on developing an effective algorithm for protein function prediction. The algorithm is based on proteins that have similar expression patterns. The similarity of the expression data is determined using a novel measure, the slope matrix. The slope matrix introduces a normalized method for the comparison of expression levels throughout a proteome. The algorithm is tested using real microarray gene expression data. Their functions are characterized using gene ontology annotations. The results of the case study indicate the protein function prediction algorithm developed is comparable to the prediction algorithms that are based on the annotations of homologous proteins. -
Case Report Full Text Online At
Case Report Full text online at http://www.jiaps.com Penile agenesis A. K. Bangroo, Ramji Khetri, Sashi Tiwari St Stephen's Hospital, Tis Hazari, Delhi Correspondence: AK Bangroo, 103, Administrative block, St. Stephens Hospital, Tis Hazari, Delhi-110054, India. E-mail: [email protected] ABSTRACT Penile agenesis is an extremely rare disorder with profound urological and psychological consequences. The goal of treatment is an early female gender assignment and feminizing reconstruction of the perineum. KEY WORDS: Aphallia, Penile agenesis, Ambiguous genitalia Penile agenesis (PA) is an extremely rare developmental the scrotal folds which were preserved for subsequent anomaly with the reported incidence of 1 in 30 million genital reconstruction. births[1]. PA is believed to result from either the absence of the genital tubercle, or its failure to develop.[2] Several DISCUSSION investigators claim the absence of corpora cavernosa and corpora spongiosum as a prerequisite for the diagnosis of The earliest case report of aphallia was by Imminger in penile agenesis.[3] Except for the reported XX-XY mosaic, 1853[2] since then only 75 cases have been reported in the patients have 46 XY karyotypes.[4] More than half of these literature[6]. Skoog and Belman[5] suggested three variants, have associated anomalies, including developmental de based on urethral position in relationship to the anal fects of the caudal axis, genitourinary and gastrointestinal sphincter, as: Postsphincteric; Presphincteric tract anomalies.[5] The scrotum, testes and testicular func (Prostatorectal fistula) and Urethral atresia. More proxi tion are usually normal[2]. mal the bladder outlet, greater is the likelihood of other anomalies and death.[5] CASE REPORT A two-day-old 3.2 kg genotypic male (46XY) neonate was brought, by a social organization, to our hospital with the complaint of absence of penis, and passage of meco nium mixed with urine through rectum. -

EAU Pocket Guidelines on Male Hypogonadism 2013
GUIDELINES ON MALE HYPOGONADISM G.R. Dohle (chair), S. Arver, C. Bettocchi, S. Kliesch, M. Punab, W. de Ronde Introduction Male hypogonadism is a clinical syndrome caused by andro- gen deficiency. It may adversely affect multiple organ func- tions and quality of life. Androgens play a crucial role in the development and maintenance of male reproductive and sexual functions. Low levels of circulating androgens can cause disturbances in male sexual development, resulting in congenital abnormalities of the male reproductive tract. Later in life, this may cause reduced fertility, sexual dysfunc- tion, decreased muscle formation and bone mineralisation, disturbances of fat metabolism, and cognitive dysfunction. Testosterone levels decrease as a process of ageing: signs and symptoms caused by this decline can be considered a normal part of ageing. However, low testosterone levels are also associated with several chronic diseases, and sympto- matic patients may benefit from testosterone treatment. Androgen deficiency increases with age; an annual decline in circulating testosterone of 0.4-2.0% has been reported. In middle-aged men, the incidence was found to be 6%. It is more prevalent in older men, in men with obesity, those with co-morbidities, and in men with a poor health status. Aetiology and forms Male hypogonadism can be classified in 4 forms: 1. Primary forms caused by testicular insufficiency. 2. Secondary forms caused by hypothalamic-pituitary dysfunction. 164 Male Hypogonadism 3. Late onset hypogonadism. 4. Male hypogonadism due to androgen receptor insensitivity. The main causes of these different forms of hypogonadism are highlighted in Table 1. The type of hypogonadism has to be differentiated, as this has implications for patient evaluation and treatment and enables identification of patients with associated health problems. -

A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years
Journal of Advances in Radiology and Medical Imaging Volume 4 | Issue 1 ISSN: 2456-5504 Case Report Open Access A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man D’Amato D*, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, Valente F and Bizzaglia M Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Rome, Italy *Corresponding author: D’Amato D, Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Viale Oxford 181, Rome, Italy, Tel: +393207034690, E-mail: [email protected] Citation: D’Amato D, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, et al. (2019) A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man. J Adv Radiol Med Image 4(1): 102 Received Date: April 04, 2019 Accepted Date: August 26, 2019 Published Date: August 28, 2019 Abstract Congenital anorchia is an uncommon clinical condition. Etiology and pathogenetic mechanisms are often unknown. Although some patients with anorchia present with ambiguous external genitalia or micropenis, most have a normal phenotype. XY Disorders of Sex Development classifications are numerous and success rate in establishing a precise diagnosis is far lower than in XX karyotype. We report the case of a young man, with 46 XY karyotype showing various uro-genital abnormalities. A definitive diagnosis was not established due to the complex clinical presentation. Ultrasonography and Magnetic Resonance Imaging techniques were useful tools in the definition of uro-genital anomalies and gonadal development in this complex scenario. Keywords: Anorchia; Cryptorchidism; Urogenital Anomalies; DSD; MRI List of abbreviations: MRI: Magnetic Resonance Imaging; US: Ultrasonography; DSD: Disorders of Sex Development, FSH: Follicle- Stimulating Hormone; HCG: Human Chorionic Gonadotropin; LH: Luteinizing Hormone; AMH Antimüllerian Hormone; LHRH LH- Releasing Hormone; SD: Standard Deviation Introduction The disorders of sexual differentiation constitute a challenging area for both diagnostic and therapeutic impact. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

(TEX) Genes: a Review Focused on Spermatogenesis and Male Fertility
Bellil et al. Basic and Clinical Andrology (2021) 31:9 https://doi.org/10.1186/s12610-021-00127-7 REVIEW ARTICLE Open Access Human testis-expressed (TEX) genes: a review focused on spermatogenesis and male fertility Hela Bellil1, Farah Ghieh2,3, Emeline Hermel2,3, Béatrice Mandon-Pepin2,3 and François Vialard1,2,3* Abstract Spermatogenesis is a complex process regulated by a multitude of genes. The identification and characterization of male-germ-cell-specific genes is crucial to understanding the mechanisms through which the cells develop. The term “TEX gene” was coined by Wang et al. (Nat Genet. 2001; 27: 422–6) after they used cDNA suppression subtractive hybridization (SSH) to identify new transcripts that were present only in purified mouse spermatogonia. TEX (Testis expressed) orthologues have been found in other vertebrates (mammals, birds, and reptiles), invertebrates, and yeasts. To date, 69 TEX genes have been described in different species and different tissues. To evaluate the expression of each TEX/tex gene, we compiled data from 7 different RNA-Seq mRNA databases in humans, and 4 in the mouse according to the expression atlas database. Various studies have highlighted a role for many of these genes in spermatogenesis. Here, we review current knowledge on the TEX genes and their roles in spermatogenesis and fertilization in humans and, comparatively, in other species (notably the mouse). As expected, TEX genes appear to have a major role in reproduction in general and in spermatogenesis in humans but also in all mammals such as the mouse. Most of them are expressed specifically or predominantly in the testis. -

Growth Hormone Deficiency Causing Micropenis:Peter A
Growth Hormone Deficiency Causing Micropenis:Peter A. Lee, MD, PhD, a Tom Mazur, PsyD, Lessons b Christopher P. Houk, MD, Learned c Robert M. Blizzard, MD d From a Well-Adjusted Adultabstract This report of a 46, XY patient born with a micropenis consistent with etiology from isolated congenital growth hormone deficiency is used to (1) raise the question regarding what degree testicular testosterone exposure aDepartment of Pediatrics, College of Medicine, Penn State to the central nervous system during fetal life and early infancy has on the University, Hershey, Pennsylvania; bCenter for Psychosexual development of male gender identity, regardless of gender of rearing; (2) Health, Jacobs School of Medicine and Biomedical suggest the obligatory nature of timely full disclosure of medical history; Sciences, University at Buffalo and John R. Oishei Children’s Hospital, Buffalo, New York; cDepartment of (3) emphasize that virtually all 46, XY infants with functional testes and Pediatrics, Medical College of Georgia, Augusta University, a micropenis should be initially boys except some with partial androgen Augusta, Georgia; and dDepartment of Pediatrics, College of Medicine, University of Virginia, Charlottesville, Virginia insensitivity syndrome; and (4) highlight the sustaining value of a positive long-term relationship with a trusted physician (R.M.B.). When this infant Dr Lee reviewed and discussed the extensive medical records with Dr Blizzard, reviewed presented, it was commonly considered inappropriate to gender assign an pertinent medical literature, and wrote each draft infant male whose penis was so small that an adult size was expected to be of the manuscript with input from all coauthors; inadequate, even if the karyotype was 46, XY, and testes were functional. -
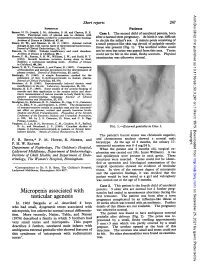
Micropenis Associated with Testicular Agenesis
Arch Dis Child: first published as 10.1136/adc.50.3.247 on 1 March 1975. Downloaded from Short reports 247 REFERENCES Patients Barnes, N. D., Joseph, J. M., Atherden, S. M. and Clayton, B. E. (1972). Functional tests of adrenal axis in children with Case 1. The second child of unrelated parents, born measurement of plasma cortisol by competitive protein binding. after a normal term pregnancy. At birth it was difficult Archives of Disease in Childhood, 47, 66. to decide the infant's sex. A minute penis consisting of Deane, H. W., and Masson, G. M. C. (1951). Adrenal cortical a small prepuce-like skin tag devoid of palpable erectile changes in rats with various types of experimental hypertension. .ournal of Clinical Endocrinology, 11, 193. tissue was present (Fig. 1). The urethral orifice could Fanconi, G. (1954). Tubular insufficiency and renal dwarfism. not be seen but urine was passed from this area. Testes Archives of Disease in Childhood, 29, 1. could not be felt in the small, fleshy scrotum. Physical Howse, P. M., Rayner, P. H. W., Williams, J. W., and Rudd, B. T. (1974). Growth hormone secretion during sleep in short examination was otherwise normal. children: a continuous sampling study. Archives of Disease in Childhood, 49, 246. James, V. H. T., Townsend, J., and Fraser, R. (1967). Comparison of fluorimetric and isotopic procedures for the determination of plasma cortisol. journal of Endocrinology, 37, xxviii. Mattingly, D. (1962). A simple fluorimetric method for the estimation of free 1 1-hydroxycorticoids in human plasma. Journal of Clinical Pathology, 15, 374. -

Constructing and Analyzing Biological Interaction Networks for Knowledge Discovery
Constructing and Analyzing Biological Interaction Networks for Knowledge Discovery Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Duygu Ucar Graduate Program in Computer Science and Engineering The Ohio State University 2009 Dissertation Committee: Srinivasan Parthasarathy, Advisor Yusu Wang Umit Catalyurek c Copyright by Duygu Ucar 2009 ABSTRACT Many biological datasets can be effectively modeled as interaction networks where nodes represent biological entities of interest such as proteins, genes, or complexes and edges mimic associations among them. The study of these biological network structures can provide insight into many biological questions including the functional characterization of genes and gene products, the characterization of DNA-protein bindings, and the under- standing of regulatory mechanisms. Therefore, the task of constructing biological interac- tion networks from raw data sets and exploiting information from these networks is critical, but is also fraught with challenges. First, the network structure is not always known in a priori; the structure should be inferred from raw and heterogeneous biological data sources. Second, biological networks are noisy (containing unreliable interactions) and incomplete (missing real interactions) which makes the task of extracting useful information difficult. Third, typically these networks have non-trivial topological properties (e.g., uneven degree distribution, small world) that limit the effectiveness of traditional knowledge discovery al- gorithms. Fourth, these networks are usually dynamic and investigation of their dynamics is essential to understand the underlying biological system. In this thesis, we address these issues by presenting a set of computational techniques that we developed to construct and analyze three specific types of biological interaction networks: protein-protein interaction networks, gene co-expression networks, and regulatory networks. -

Making Ribosomes: Biochemical and Structural Studies of Early Ribosome Biogenesis in Yeast Malik Chaker-Margot
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2018 Making Ribosomes: Biochemical and Structural Studies of Early Ribosome Biogenesis in Yeast Malik Chaker-Margot Follow this and additional works at: https://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons Recommended Citation Chaker-Margot, Malik, "Making Ribosomes: Biochemical and Structural Studies of Early Ribosome Biogenesis in Yeast" (2018). Student Theses and Dissertations. 472. https://digitalcommons.rockefeller.edu/student_theses_and_dissertations/472 This Thesis is brought to you for free and open access by Digital Commons @ RU. It has been accepted for inclusion in Student Theses and Dissertations by an authorized administrator of Digital Commons @ RU. For more information, please contact [email protected]. MAKING RIBOSOMES: BIOCHEMICAL AND STRUCTURAL STUDIES OF EARLY RIBOSOME BIOGENESIS IN YEAST A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy by Malik Chaker-Margot June 2018 © Copyright by Malik Chaker-Margot 2018 MAKING RIBOSOMES: BIOCHEMICAL AND STRUCTURAL STUDIES OF EARLY RIBOSOME BIOGENESIS IN YEAST Malik Chaker-Margot, Ph.D. The Rockefeller University 2018 The ribosome is a complex macromolecule responsible for the synthesis of all proteins in the cell. In yeast, it is made of four ribosomal RNAs and 79 proteins, asymmetrically divided in a small and large subunit. In a growing yeast cell, more than 2000 ribosomes are assembled every minute. The ribosome is assembled through a highly complex process involving more than 200 trans-acting factors. Ribosome assembly begins in the nucleolus where RNA polymerase I transcribes a long polycistronic RNA, the 35S pre- ribosomal RNA which contains the sequences for three of the four ribosomal RNAs, as well as spacer sequences which are transcribed and removed during assembly. -

Intersex, Discrimination and the Healthcare Environment – a Critical Investigation of Current English Law
Intersex, Discrimination and the Healthcare Environment – a Critical Investigation of Current English Law Karen Jane Brown Submitted in Partial Fulfilment of the Requirements of London Metropolitan University for the Award of PhD Year of final Submission: 2016 Table of Contents Table of Contents......................................................................................................................i Table of Figures........................................................................................................................v Table of Abbreviations.............................................................................................................v Tables of Cases........................................................................................................................vi Domestic cases...vi Cases from the European Court of Human Rights...vii International Jurisprudence...vii Tables of Legislation.............................................................................................................viii Table of Statutes- England…viii Table of Statutory Instruments- England…x Table of Legislation-Scotland…x Table of European and International Measures...x Conventions...x Directives...x Table of Legislation-Australia...xi Table of Legislation-Germany...x Table of Legislation-Malta...x Table of Legislation-New Zealand...xi Table of Legislation-Republic of Ireland...x Table of Legislation-South Africa...xi Objectives of Thesis................................................................................................................xii