662510V1.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Behavioral Plasticity Through the Modulation of Switch Neurons
Behavioral Plasticity Through the Modulation of Switch Neurons Vassilis Vassiliades, Chris Christodoulou Department of Computer Science, University of Cyprus, 1678 Nicosia, Cyprus Abstract A central question in artificial intelligence is how to design agents ca- pable of switching between different behaviors in response to environmental changes. Taking inspiration from neuroscience, we address this problem by utilizing artificial neural networks (NNs) as agent controllers, and mecha- nisms such as neuromodulation and synaptic gating. The novel aspect of this work is the introduction of a type of artificial neuron we call \switch neuron". A switch neuron regulates the flow of information in NNs by se- lectively gating all but one of its incoming synaptic connections, effectively allowing only one signal to propagate forward. The allowed connection is determined by the switch neuron's level of modulatory activation which is affected by modulatory signals, such as signals that encode some informa- tion about the reward received by the agent. An important aspect of the switch neuron is that it can be used in appropriate \switch modules" in or- der to modulate other switch neurons. As we show, the introduction of the switch modules enables the creation of sequences of gating events. This is achieved through the design of a modulatory pathway capable of exploring in a principled manner all permutations of the connections arriving on the switch neurons. We test the model by presenting appropriate architectures in nonstationary binary association problems and T-maze tasks. The results show that for all tasks, the switch neuron architectures generate optimal adaptive behaviors, providing evidence that the switch neuron model could be a valuable tool in simulations where behavioral plasticity is required. -

A Model of Long-Term Memory Storage in the Cerebellar Cortex: a Possible Role for Plasticity at Parallel Fiber Synapses Onto Stellate͞basket Interneurons
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14200–14205, December 1997 Psychology A model of long-term memory storage in the cerebellar cortex: A possible role for plasticity at parallel fiber synapses onto stellateybasket interneurons GARRETT T. KENYON† Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, 6431 Fannin, Houston, TX 77030 Edited by Richard F. Thompson, University of Southern California, Los Angeles, CA, and approved October 3, 1997 (received for review April 14, 1997) ABSTRACT By evoking changes in climbing fiber activity, Despite evidence supporting their postulated role in motor movement errors are thought to modify synapses from parallel learning, theoretical arguments suggest that memories stored fibers onto Purkinje cells (pf*Pkj) so as to improve subse- at pf*Pkj synapses would be susceptible to long-term degra- quent motor performance. Theoretical arguments suggest dation as a result of ongoing motor adaptation. First, motor there is an intrinsic tradeoff, however, between motor adap- memories are likely to be distributed across overlapping sets of tation and long-term storage. Assuming a baseline rate of pf*Pkj synapses. If motor memories did not overlap, the motor errors is always present, then repeated performance of storage capacity of pf*Pkj synapses would be greatly reduced any learned movement will generate a series of climbing (12, 17). Second, it is reasonable to assume that the pattern of fiber-mediated corrections. By reshuffling the synaptic synaptic weights necessary to effect any given movement is weights responsible for any given movement, such corrections non-unique. If the activity of a Purkinje cell depends only on will degrade the memories for other learned movements stored the total sum of the synaptic input at any given moment, then in overlapping sets of synapses. -

How Local Excitation-Inhibition Ratio Impacts the Whole Brain Dynamics Gustavo Deco Universitat Pompeu Fabra
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2014 How local excitation-inhibition ratio impacts the whole brain dynamics Gustavo Deco Universitat Pompeu Fabra Adrian Ponce-Alvarez Universitat Pompeu Fabra Patric Hagmann University of Lausanne Gian Luca Romani G. d'Annunzio University Dante Mantini University of Oxford See next page for additional authors Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Deco, Gustavo; Ponce-Alvarez, Adrian; Hagmann, Patric; Romani, Gian Luca; Mantini, Dante; and Corbetta, Maurizio, ,"How local excitation-inhibition ratio impacts the whole brain dynamics." The ourJ nal of Neuroscience.34,23. 7886-7898. (2014). https://digitalcommons.wustl.edu/open_access_pubs/2973 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Authors Gustavo Deco, Adrian Ponce-Alvarez, Patric Hagmann, Gian Luca Romani, Dante Mantini, and Maurizio Corbetta This open access publication is available at Digital Commons@Becker: https://digitalcommons.wustl.edu/open_access_pubs/2973 7886 • The Journal of Neuroscience, June 4, 2014 • 34(23):7886–7898 Systems/Circuits How Local Excitation–Inhibition Ratio Impacts the Whole Brain Dynamics Gustavo Deco,1,2* Adria´n Ponce-Alvarez,1* Patric Hagmann,3,4 Gian Luca Romani,5 -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -

The Stellate Cells of the Rat's Cerebellar Cortex*
Z. Anat. Entwickl.-Gesch. 136, 224--248 (1972) by Springer-Verlag 1972 The Stellate Cells of the Rat's Cerebellar Cortex* Victoria Chan-Palay and Sanford L. Palay Departments of Neurobiology and Anatomy, Harvard Medical School, Boston, Massachusetts Received February 18, 1972 Summary. Stellate cells were studied in rapid Golgi preparations and in electron micro- graphs. These small neurons can be classified on the basis of their position in the molecular layer and the patterns of their dendritic and axonal arborizations as follows: (1) superficial cells with short, contorted dendrites and a circumscribed axonal arbor (upper third of the molecular layer) ; (2) deep stellate cells with radiating, twisted dendrites and with long axons giving rise to thin, varicose collaterals (middle third of the molecular layer); (3) deep stellate cells with similar dendrites and long axons giving collaterals to the basket around the Purkinje cell bodies (middle third of the molecular layer). An important characteristic of the stellate cell axon is that it generates most of its collaterals close to its origin. Even in long axon cells, only a few collaterals issue from the more distant parts of the axon. These forms contrast with the basket cell, which sends out long, straighter dendrites, and an extended axon that first emits branches at some distance from its origin. Furthermore, basket cell axon collaterals are usually stout in contrast to the frail, beaded collaterals of the stellate cell axon. The two cell types are considered to be distinct. In electron micrographs stellate cells display folded nuclei and sparse cytoplasm with the characteristics usual for small neurons. -

Context-Modular Memory Networks Support High-Capacity, Flexible, And
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.08.898528; this version posted January 9, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Context-modular memory networks support high-capacity, flexible, and robust associative memories William F. Podlaski,∗ Everton J. Agnes,y and Tim P. Vogelsy Centre for Neural Circuits and Behaviour, University of Oxford, OX1 3SR Oxford, United Kingdom Context, such as behavioral state, is known to modulate memory formation and retrieval, but is usually ignored in associative memory models. Here, we propose several types of contextual modulation for associative memory networks that greatly increase their performance. In these networks, context inactivates specific neurons and connections, which modulates the effective connectivity of the network. Memories are stored only by the active components, thereby reducing interference from memories acquired in other contexts. Such networks exhibit several beneficial characteristics, including enhanced memory capacity, high robustness to noise, increased robustness to memory overloading, and better memory retention during continual learning. Furthermore, memories can be biased to have different relative strengths, or even gated on or off, according to contextual cues, providing a candidate model for cognitive control of memory and efficient memory search. An external context-encoding network can dynamically switch the memory network to a desired state, which we liken to experimentally observed contextual signals in prefrontal cortex and hippocampus. Overall, our work illustrates the benefits of organizing memory around context, and provides an important link between behavioral studies of memory and mechanistic details of neural circuits. -

Memory Trace Replay: the Shaping of Memory Consolidation by Neuromodulation
Atherton, L. A., Duprett, D., & Mellor, J. R. (2015). Memory trace replay: The shaping of memory consolidation by neuromodulation. Trends in Neurosciences, 38(9), 560-570. https://doi.org/10.1016/j.tins.2015.07.004 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1016/j.tins.2015.07.004 Link to publication record in Explore Bristol Research PDF-document University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Review Memory trace replay: the shaping of memory consolidation by neuromodulation 1 2 1 Laura A. Atherton , David Dupret , and Jack R. Mellor 1 School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK 2 Medical Research Council Brain Network Dynamics Unit at the University of Oxford, Department of Pharmacology, Oxford, OX1 3TH, UK The consolidation of memories for places and events is (sSWRs), long periods of awake immobility (iSWRs), or thought to rely, at the network level, on the replay of brief pauses in exploration (eSWRs) (reviewed in [9,10]). spatially tuned neuronal firing patterns representing Sequential replay can occur in both a forward [11–15] discrete places and spatial trajectories. This occurs in and backward [11,13–16] direction, with the directional the hippocampal-entorhinal circuit during sharp wave balance proposed to be dependent on the ongoing behav- ripple events (SWRs) that occur during sleep or rest. -

Context-Modular Memory Networks Support High-Capacity, Flexible, And
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.08.898528; this version posted January 9, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Context-modular memory networks support high-capacity, flexible, and robust associative memories William F. Podlaski,∗ Everton J. Agnes,y and Tim P. Vogelsy Centre for Neural Circuits and Behaviour, University of Oxford, OX1 3SR Oxford, United Kingdom Context, such as behavioral state, is known to modulate memory formation and retrieval, but is usually ignored in associative memory models. Here, we propose several types of contextual modulation for associative memory networks that greatly increase their performance. In these networks, context inactivates specific neurons and connections, which modulates the effective connectivity of the network. Memories are stored only by the active components, thereby reducing interference from memories acquired in other contexts. Such networks exhibit several beneficial characteristics, including enhanced memory capacity, high robustness to noise, increased robustness to memory overloading, and better memory retention during continual learning. Furthermore, memories can be biased to have different relative strengths, or even gated on or off, according to contextual cues, providing a candidate model for cognitive control of memory and efficient memory search. An external context-encoding network can dynamically switch the memory network to a desired state, which we liken to experimentally observed contextual signals in prefrontal cortex and hippocampus. Overall, our work illustrates the benefits of organizing memory around context, and provides an important link between behavioral studies of memory and mechanistic details of neural circuits. -

The Developmental Genetics of the Cerebellum and the Genetic Bases of Known Cerebellar Disorders
THE DEVELOPMENTAL GENETICS OF THE CEREBELLUM AND THE GENETIC BASES OF KNOWN CEREBELLAR DISORDERS A Review of Developmental Genetics of the Cerebellum and the Genetic Bases of known Cerebellar Disorders Olakada Favour Adamba 17/MHS01/254 Department of Medicine and Health Sciences College of Medicine and Health Sciences Afe Babalola University ANA 303: Neuroanatomy July, 2020 The Developmental Genetics of Cerebellum and the Genetic Bases of known Cerebellar Disorders: A Literature Review Olakada Favour Adamba1 Abstract The internal structure of the cerebellum is an intriguing paradox; its cytoarchitecture is relatively simple compared to the connections between its neurons, which are wired into a complex array of gene expression domains and functional circuits. The genetic research of cerebellar development has provided a great deal of information about the molecular events directing the formation of the cerebellum. The developmental mechanisms that coordinate the establishment of cerebellar structure and circuitry provide a powerful model for understanding how a functional brain develops and its significance in cerebellar disorders and diseases. The cellular makeup of the cerebellum is derived from two primary germinal matrices (the ventricular zone and a specialized germinal matrix called the rhombic lip). Each matrix/zone expresses a specific set of genes that establish the cell lineages within the cerebellar anlage. Then, cohorts of differentiated projection neurons and interneuron progenitors migrate into the developing cerebellum. thereafter, a number of remarkable patterning events occur. Altogether, structural and molecular organisations are thought to support the proper connectivity between incoming afferent projections and their target cells. Key words: Cerebellum, circuitry, genetic, development, disorders. I. Introduction The cerebellum (‘little brain’) resides at the anterior end of the hindbrain and is classically defined by its role in sensory-motor processing (Buckner, 2013). -
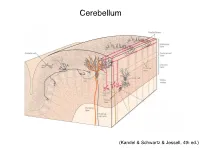
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

An Updated Investigation on the Dromedary Camel Cerebellum (Camelus Dromedarius) with Special Insight Into the Distribution of Calcium‑Binding Proteins Abdelraheim H
www.nature.com/scientificreports OPEN An updated investigation on the dromedary camel cerebellum (Camelus dromedarius) with special insight into the distribution of calcium‑binding proteins Abdelraheim H. Attaai1,3, Ahmed E. Noreldin2, Fatma M. Abdel‑maksoud1* & Manal T. Hussein1,3 Studying the cerebella of diferent animals is important to expand the knowledge about the cerebellum. Studying the camel cerebellum was neglected even though the recent research in the middle east and Asia. Therefore, the present study was designed to achieve a detailed description of the morphology and the cellular organization of the camel cerebellum. Because of the high importance of the calcium ions as a necessary moderator the current work also aimed to investigate the distribution of calcium binding proteins (CaBP) such as calbindin D‑28K (CB), parvalbumin (PV) and calretinin (CR) in diferent cerebellar cells including the non‑traditional neurons. The architecture of camel cerebellum, as diferent mammals, consists of the medulla and three layered‑cortex. According to our observation the cells in the granular layer were not crowded and many spaces were observed. CB expression was the highest by Purkinje cells including their dendritic arborization. In addition to its expression by the inhibitory interneurons (basket, stellate and Golgi neurons), it is also expressed by the excitatory granule cells. PV was expressed by Purkinje cells, including their primary arborization, and by the molecular layer cells. CR immunoreactivity (‑ir) was obvious in almost all cell layers with varying degrees, however a weak or any expression by the Purkinje cells. The molecular layer cells and the Golgi and the non traditional large neurons of the granular layer showed the strongest CR‑ir. -

Spontaneous Activity Signatures of Morphologically Identified Interneurons in the Vestibulocerebellum
712 • The Journal of Neuroscience, January 12, 2011 • 31(2):712–724 Behavioral/Systems/Cognitive Spontaneous Activity Signatures of Morphologically Identified Interneurons in the Vestibulocerebellum Tom J. H. Ruigrok,1 Robert A. Hensbroek,2 and John I. Simpson2 1Department of Neuroscience, Erasmus Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands, and 2Department of Physiology and Neuroscience, New York University School of Medicine, New York, New York 10016 Cerebellar cortical interneurons such as Golgi cells, basket cells, stellate cells, unipolar brush cells, and granule cells play an essential role in the operations of the cerebellum. However, detailed functional studies of the activity of these cells in both anesthetized and behaving animals have been hampered by problems in recognizing their physiological signatures. We have extracellularly recorded the spontane- ous activity of vestibulocerebellar interneurons in ketamine/xylazine-anesthetized rats and subsequently labeled them with Neurobiotin using the juxtacellular technique. After recovery and morphological identification of these cells, they were related to statistical measures of their spontaneous activity. Golgi cells display a somewhat irregular firing pattern with relatively low average frequencies. Unipolar brush cells are characterized by more regular firing at higher rates. Basket and stellate cells are alike in their firing characteristics, which mainly stand out by their irregularity; some of them are set apart by their very slow average rate. The spontaneous activity of interneurons examined in the ketamine/xylazine rabbit fit within this general pattern. In the rabbit, granule cells were identified by the spontaneous occurrence of extremely high-frequency bursts of action potentials, which were also recognized in the rat.