Biodegradation of Petroleum Hydrocarbons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inventory of Environmental Work in China
INVENTORY OF ENVIRONMENTAL WORK IN CHINA In this fifth issue of the China Environment Series, the Inventory of Environmental Work in China has been updated and we made extra effort to add many new groups, especially in the Chinese organization section. To better highlight the growing number of U.S. universities and professional associations active in China we have created a separate section. In the past inventories we have gathered information from U.S. government agencies; from this year forward we will be inventorying the work done by other governments as well. This inventory aims to paint a clearer picture of the patterns of aid and investment in environmental protection and energy-efficiency projects in the People’s Republic of China. We highlight a total of 118 organizations and agencies in this inventory and provide information on 359 projects. The five categories of the inventory are listed below: Part I (p. 138): United States Government Activities (15 agencies/organizations, 103 projects) Part II (p. 163): U.S. and International NGO Activities (33 organizations, 91 projects) Part III (p. 190): U.S. Universities and Professional Association Activities (9 institutions, 27 projects) Part IV (p. 196): Chinese and Hong Kong NGO and GONGO Activities (50 organizations, 61 projects) Part V (p. 212): Bilateral Government Activities (11 agencies/organizations, 77 projects) Since we have expanded the inventory, even more people than last year contributed to the creation of this inventory. We are grateful to all of those in U.S. government agencies, international and Chinese nongovernmental organizations, universities, as well as representatives in foreign embassies who generously gave their time to compile and summarize the information their organizations and agencies undertake in China. -

2010 Sustainability Report
2010 Sustainability Report 1 From the Chairman of the Board 2 Dialogue with the President 4 Energy Outlook 6 About This Report About Us 8 Facts of 2010 12 Stakeholders 14 PetroChina Company Limited (PetroChina, also hereinafter referred to as the “Company” “we” and “us”) has published its annual Sustainable Energy Supplies Sustainability Report since 2006. This is to present our stakeholders and the public with a clear picture of our commitment and performance in pursuit of scientific, safe, clean, economical and harmonious development. We also hope the Company can win 1.Exploration and Development 17 wider support and regard from various social communities. 2. Refining and Chemicals 20 This report represents what we did to honour our commitments to the economy, environment and society in 2010. All information 3. International Cooperation 21 disclosed in the report was sourced from PetroChina’s official documents, statistics reports as well as from statistics gathered from 4. Oil and Gas Supply 22 the Company’s affiliated enterprises, and has been reviewed in accordance with the Company’s Rules for Information Disclosure 5. New Energies 25 Control and Disclosure Procedures. Given continuity and comparability, the report provides historical and future explanations on certain issues. The report is formulated according to the principles of accuracy, materiality, standardisation and transparency. We also referred to Safe and Clean Production and Operation the Guideline on Preparing the Report on Performance of Corporate Social Responsibility by the Shanghai Stock Exchange and continue to consult the Sustainability Reporting Guideline released by the Global Reporting Initiative in 2006 and the Oil and Gas Industry 1. -

Crude Beginnings: Investor Brief
Crude beginnings: Investor brief An assessment of China National Petroleum Corporation’s environmental and social performance abroad With contributions from The Altai Project, AmazonWatch, Conflict Risk Network, Fund for 21st Century Altai, Pacific Environment, Revenue Watch Institute, Save Ukok Coalition and Shwe Gas Movement Crude beginnings: An assessment of China National Petroleum Corporation’s environmental and social performance abroad Author: Adina Matisoff, Friends of the Earth For more information or for copies of the report contact: Michelle Chan Friends of the Earth 311 California St., Ste. 510 San Francisco, CA 94104 415.544.0790 (p) [email protected] We gratefully acknowledge the generous support of the Ford Foundation, the CS Mott Foundation, the Rockefeller Brothers Fund and the JMG Fund. The views expressed in this report are those of Friends of the Earth and do not necessarily represent those of contributing writers, individual contributors to Friends of the Earth or the funding organizations. February 2012 Table of contents Executive summary 3 Introduction 6 CNPC in the world 7 Company overview 7 Going global 7 Motivations for going abroad 8 Encountering risks 9 Future plans 9 CNPC & environmental and social governance 10 CNPC’s internal policies and management structures 10 Government oversight relevant to CNPC 16 Input from research institutes and academics 22 The Chinese media 24 Additional sources of corporate governance oversight 24 CNPC & environmental and social performance 29 Stakeholder engagement 29 Environmental -
Summary Environmental Impact Assessment Zhengzhou-Xi'an
SUMMARY ENVIRONMENTAL IMPACT ASSESSMENT ZHENGZHOU-XI’AN RAILWAY PROJECT IN THE PEOPLE’S REPUBLIC OF CHINA April 2005 CURRENCY EQUIVALENTS (as of 12 April 2005) Currency Unit – yuan (CNY) CNY1.00 = $0.1208 $1.00 = CNY8.2775 ABBREVIATIONS ADB – Asian Development Bank BOD – biochemical oxygen demand CO – carbon monoxide COD – chemical oxygen demand dB – decibel EIA – environmental impact assessment EMP – environmental management plan EMU – electric motor unit EPB – Local Environmental Protection Bureau FSDI – Fourth Survey and Design Institute of Railways GDP – gross domestic product JVC - joint venture company MOR – Ministry of Railways MWh – megawatt-hour NGO – nongovernment organization NOx – oxides of nitrogen NO2 – nitrogen dioxide PPTA – project preparatory technical assistance PRC – People’s Republic of China SBR – sequencing batch reactor SEPA – State Environmental Protection Agency SO2 – sulfur dioxide SOx – oxides of sulfur SS – suspended solids TERA – Transportation and Economic Research Associates THC – total hydrocarbon TSP – total suspended particulates VOC – volatile organic compound WWF – World Wildlife Federation ZXR – Zhengzhou–Xi’an Railway WEIGHTS AND MEASURES C – centigrade cm – centimeter ha – hectare kg – kilogram km – kilometer m – meter mg/l – milligram per liter mg/m3 – milligram per cubic meter m2 – square meter m3 – cubic meter mm – millimeter m/s – meter per second mu – a unit of area (=666.7 m2) MWh – megawatt-hour ha – hectare (1 hectare=15 mu) nm3 /a – cubic meter (normalized) per annum t/a – ton per annum NOTE In this report, “$” refers to US dollars CONTENTS Page MAPS I. INTRODUCTION 1 II. DESCRIPTION OF THE PROJECT 1 III. DESCRIPTION OF THE ENVIRONMENT 2 A. PHYSICAL ENVIRONMENT 2 B. -
Chapter 1 General Introduction and Review of Literature
Chapter 1 General introduction and review ofliterature 1.1. Introduction Every year spillage of tones of fuel and lubricating oil has become a major environmental hazard. In October 20 I 4, a single oil spillage of 546 tones was reported at Mid-Valley Pipeline of Louisiana, United States. A recent incident highlighted how time consuming a cleanup operation of oil spillage can be. According to CNN International, in Nov 2013, oil-spillage occurred in Phillipines due to a typhoon and till date the cleaning up process is going on. There are also long-term effects on ecosystems related to the release of toxic components over a prolonged period as the oil breaks up and the concentration of toxicants in organisms towards the top of the food chain increases (Lin et al., 2008). These contaminations of the environment with petroleum hydrocarbons provides serious problems for many countries including India. Release of petroleum hydrocarbons into the environment whether accidentally or due to human activities, is a main cause of water and soil pollution. Soil contamination with hydrocarbons cause extensive damage to local system as accumulation of pollutants in animals and plant tissues results in death or mutations (Mehrasbi et a/.,2003 and Head et al., 2006). Several physical, chemical and thermal methods have commonly been employed to clean up the oil-contaminated sites (Frick et a/., 1999). However, these techniques are relatively expensive and also require site restoration (Lundstedt et al., 2003). Biodegradation of hydrocarbon-contaminated soils exploits the ability of microorganisms to degrade or detoxifY organic contamination. It has been established as an efficient, economic, versatile and environmentally sound treatment (Bundy et a/., 2004).Microorganisms that biodegrade the components of petroleum hydrocarbons are isolated from various environments, particularly from petroleum-contaminated sites (Ghanavati et al., 201 0). -

Situation Analysis of Shaanxi Province
China Climate Change Partnership Framework - Enhanced strategies for climate-proofed and environmentally sound agricultural production in the Yellow River Basin (C-PESAP) Situation Analysis of Shaanxi Province Compiled and Written by MIN Ancheng & HAN Qinfang Checked by JIA Zhikuan Northwest Agriculture and Forestry University Yangling, Shaanxi, P R China Situation Analysis of Shaanxi Province Contents 1 Introduction......................................................................................................................................... 1 1.1 Background and rationale of the study ........................................................................................ 1 1.1.1 Background of climate change............................................................................................ 1 1.1.2 Impact of climate change on agriculture in the Yellow River Basin...................................2 1.1.3 Project Origin......................................................................................................................3 1.2 General description of the study area........................................................................................... 4 1.2.1 The Yellow River Basin ......................................................................................................4 1.2.2 Shaanxi................................................................................................................................6 1.2.2.1 Profile........................................................................................................................7 -

China and the Global Environment LEARNING from the PAST, ANTICIPATING the FUTURE Katherine Morton First Published for Lowy Institute for International Policy 2009
Lowy Institute Paper 29 China and the global environment LEARNING FROM THE PAST, ANTICIPATING THE FUTURE Katherine Morton First published for Lowy Institute for International Policy 2009 PO Box 102 Double Bay New South Wales 1360 Australia www.longmedia.com.au [email protected] Tel. (+61 2) 9362 8441 Lowy Institute for International Policy © 2009 ABN 40 102 792 174 All rights reserved. Without limiting the rights under copyright reserved above, no part Katherine Morton is a Fellow in the Department of of this publication may be reproduced, stored in or introduced into a retrieval system, International Relations, College of Asia and the Pacific, or transmitted in any form or by any means (including but not limited to electronic, The Australian National University. She is a specialist on mechanical, photocopying, or recording), without the prior written permission of the China and Global Politics with over a decade of experience copyright owner. working on environmental problems at the local, national, Photos © Katherine Morton copyright 2009 and international levels. For the past six years, she has been Maps © ANU cartography copyright 2009 conducting research on the Tibetan Plateau looking at the impacts of climate change on local communities as well Cover design by Longueville Media/Nina Nielsen as the implications for regional security. She is currently Typeset by Longueville Media in Esprit Book 10/13 working with international partners to develop a visual National Library of Australia Cataloguing-in-Publication entry cartography of climate related risks across the Greater Author: Morton, Katherine. Himalayas. Dr Morton is also the Chief Investigator in a Title: China and the global environment : learning from the past, Ford Foundation funded collaborative research project on anticipating the future / Katherine Morton. -

West-East Gas Pipeline Project ( 2002-2013 ) Special Report on Social Responsibility
West-East Gas Pipeline Project ( 2002-2013 ) Special Report on Social Responsibility Caring for Energy· Caring for You www.cnpc.com.cn West-East Gas Pipeline Project (2002-2013) Special Report on Social Responsibility China National Petroleum Corporation China National Petroleum Corporation Recycled/Recyclable Contents 16 Pipeline Construction and Operation 18 Safe and Environmentally Friendly Engineering 22 Securing Gas Supply 23 Ensuring Operational Safety 6 Overview of the West-East Gas Pipeline Project 2 Message from the Chairman 35 Appendix 35 Milestones 40 Terminology 34 Outlook Promoting Industrial 8 Improving Energy 26 Development Pattern 28 Boosting Industrial Upgrading 10 Guaranteeing Energy 31 Driving Local Development Security 33 Opening up and Cooperation 11 Optimizing Energy Mix for Common Development 4 West-East Gas Pipelines and 12 Improving the Natural Gas Utilization in China Environment 14 Enhancing Quality of Life 2 West-East Gas Pipeline Project (2002-2013) Special Report on Social Responsibility China National Petroleum Corporation 3 Message from the Chairman As we are confronted with pressing challenges stemming also put emphasis on the development and utilization of coal- development. During the construction of the pipelines, we from the growing contradiction between energy bed methane, shale gas and other unconventional gases, and strictly controlled project quality, minimized the impact on the demand and environmental protection, we are in need continue to secure safe and reliable gas supply with flexible environment and communities, and protected the ecological of the transformation to green energies now more than scheduling and peak shaving capacities. In China, we have built environment and cultural relics along the lines. -

Green Transition of Cultivated Land Use in the Yellow River Basin: a Perspective of Green Utilization Efficiency Evaluation
land Article Green Transition of Cultivated Land Use in the Yellow River Basin: A Perspective of Green Utilization Efficiency Evaluation Xiao Lu 1,2,*, Yi Qu 2, Piling Sun 2, Wei Yu 2 and Wenlong Peng 2 1 School of Humanities and Law, Northeastern University, Shenyang 110169, China 2 School of Geography and Tourism, Qufu Normal University, Rizhao 276826, China; [email protected] (Y.Q.); [email protected] (P.S.); [email protected] (W.Y.); [email protected] (W.P.) * Correspondence: [email protected]; Tel.: +86-187-6931-9508 Received: 1 November 2020; Accepted: 24 November 2020; Published: 27 November 2020 Abstract: Exploring the green transition of cultivated land use from the perspective of green utilization efficiency evaluation has become an important content of deepening the study of cultivated land use transition, which is of great significance to promote food security and ecological civilization construction. At present, there are few studies on the green utilization efficiency of cultivated land (GUECL), which covers the comprehensive benefits of economy, ecology and society, combined with the requirements of ecological civilization and green development. Taking 65 cities (regions and autonomous prefectures) of the Yellow River Basin as the basic evaluation unit, the GUECL of the Yellow River Basin is evaluated with a Super-SBM model. In general, the GUECL of the Yellow River Basin was not high at four time points of 2000, 2006, 2012 and 2018, which presents a trend of “rising first and then falling”. Analyzing its temporal and spatial evolution pattern, the GUECL in the upper, middle and lower reaches presented an order of the upper reaches area > the lower reaches area > the middle reaches area; and the spatial variation trend showed a decrease from west to east, and a U-shaped change in the south-north direction. -

2014 Sustainability Report 2014 Sustainability Report 3
CONTENTS SUSTAINED ENERGY SAFE AND CLEAN CUSTOMER-FIRST “PEOPLE-ORIENTED” GIVING BACK SUPPLY PRODUCTION AND MARKET SERVICE EMPLOYEE DEVELOPMENT TO SOCIETY OPERATION 02 About PetroChina 19 Consolidating Our Resource Base 25 Improving HSE Management 37 Enhancing Quality Management 45 Employee Rights 53 Promoting Local Development 04 Message from the Chairman 20 Optimizing Our Product Mix and 28 Enhancing Operational Safety 39 Focusing on Product Innovation 47 Occupational Health Refining Geographical Coverage 54 Poverty Alleviation 06 Interview with the President 30 Ecological Protection 40 Providing High-Quality Products 48 Platform for Employee 20 Pushing Forward Pipeline Development 55 Supporting Education Promoting Energy Saving and Delivering Considerate Services 08 Highlights in 2014 Network Construction 33 42 Emission Reduction 51 Local Staff Development 56 Volunteer Activities 43 Ensuring Stable Supply 10 Corporate Governance 21 Developing Alternative Energy 34 Response to Climate Change 51 Security in Overseas Operations 57 Contributions to Overseas 15 Communication with 23 Deepening International Communities Stakeholders Cooperation 60 Investor FAQs 64 Objectives and Plans 66 Performance Statistics 68 Approach to Reporting About PetroChina Core Business PetroChina Company Limited (PetroChina, hereinafter also referred Our to as “the Company”, “we” or “us”) was established on 5 November, Energize, Harmonize, Realize Upstream Oil and Gas Exploration, Development and Production 1999, upon the restructuring of China National Petroleum Mission Our upstream Corporation (CNPC). PetroChina was listed on the NYSE (ADS business includes PetroChina takes a leading position in domestic oil and gas exploration and code: PRT) and the HKEx (stock code: 00857) in April 2000 and on Our To be a world-class international the exploration, development business. -

Study on the Spatial Distribution of Water Resource Value in the Agricultural System of the Yellow River Basin
© 2021 The Authors Water Policy Vol 23 No 4, 1044 doi: 10.2166/wp.2021.176 Study on the spatial distribution of water resource value in the agricultural system of the Yellow River Basin Huiliang Wanga,b, Shuoqiao Huangc, Danyang Dib, Yu Wanga,* and Fengyi Zhangb a Yellow River Engineering Consulting Co., Ltd., Zhengzhou 450003, China b School of Water Conservancy Engineering, Zhengzhou University, Zhengzhou 450001, China c Chengxi Water Conservancy Service Center Station, Liandu 323000, China *Corresponding author. E-mail: [email protected] ABSTRACT To analyze the spatial distribution characteristics of water resource value in the agricultural system of the Yellow River Basin, this paper takes the Yellow River Basin as its research object and studies the spatial distribution characteristics and influencing factors of water resource value in the agricultural system using the emergy theory and method, the spatial autocorrelation analysis method, and the spatial regression model. The results show the following. (1) The value of water resources in the agri- cultural system ranges from 0.64 to 0.98 $/m3, and the value in the middle and lower reaches of the basin is relatively high. (2) The Moran index of the water resource value in the agricultural system is 0.2772, showing a positive spatial autocorrelation feature. Here, ‘high-high (high value city gathering)’ is the main aggregation mode, which is mainly concentrated in the middle and lower reaches of the basin. (3) The spatial error model, moreover, has the best simulation effect. The cultivated land area, total agricultural output value, agricultural labor force, and total mechanical power have a significant positive impact on the agricultural production value of water resources in the Yellow River Basin; the altitude, annual average temperature, and agri- cultural water consumption have a negative impact. -
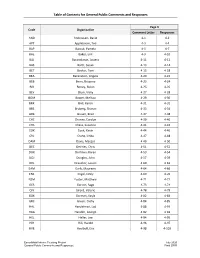
General Public Comments and Responses
Table of Contents for General Public Comments and Responses Page # Code Organization Comment Letter Responses AND Andreasen, David 4-1 4-2 APT Applebaum, Ted 4-3 4-4 BAP Baczuk, Pamela 4-5 4-7 BAE Baker, Erik 4-9 4-10 BAJ Barandiaran, Javiera 4-11 4-12 BAS Bartz, Susan 4-13 4-14 BET Becker, Tom 4-15 4-18 BEA Berenstein, Angela 4-20 4-22 BEB Berry, Bryanna 4-23 4-24 BIR Birney, Robin 4-25 4-26 BLV Blum, Vicky 4-27 4-28 BOM Bower, Melissa 4-29 4-30 BRK Brill, Karen 4-31 4-32 BRS Broberg, Sharon 4-33 4-34 BRB Brown, Brad 4-37 4-38 CHC Chanev, Carolyn 4-39 4-40 CHS Chess, Susanne 4-41 4-43 COK Cook, Kevin 4-44 4-46 CRL Crane, Linda 4-47 4-48 DAM Davis, Margot 4-49 4-50 DEC DeVries, Chris 4-51 4-52 DOK Dorfman, Karen 4-53 4-54 DOJ Douglas, John 4-57 4-59 DRL Draucker, Louise 4-60 4-62 EAM Earls, Maureen 4-64 4-66 ENE Engel, Emily 4-69 4-70 FOM Foster, Matthew 4-71 4-72 GES Gerson, Sage 4-73 4-74 GIV Girard, Valerie 4-78 4-79 GOK Gorman, Kayla 4-82 4-83 GRC Green, Cathy 4-84 4-85 HAL Handelman, Lad 4-88 4-91 HAG Handler, George 4-92 4-93 HEL Heller, Lee 4-94 4-95 HIH Hill, Harold 4-96 4-97 HVE Hvolboll, Eric 4-98 4-103 ExxonMobil Interim Trucking Project July 2020 General Public Comments and Responses Final SEIR Table of Contents for General Public Comments and Responses Page # Code Organization Comment Letter Responses JOM Jones, Melanja 4-106 4-107 KIB Kieler, Berna 4-110 4-111 KIG Kilfoyle, Gwendolyn 4-114 4-115 LAA Lagerquist, Amy 4-118 4-119 LEB Leonard, Brendan 4-123 4-124 LIK Libis, Kyle 4-128 4-129 LUJ Lumley, Julie 4-130 4-131 MAK