Changes in the Composition and Distribution of Alien Plants in South Africa: an Update from the Southern African Plant Invaders Atlas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

NOTES Watsonia 25 (2005) NORTH WALES SPECIES of RUBUS L
Watsonia 25: 289–298 (2005) NOTES Watsonia 25 (2005) 289 Notes NORTH WALES SPECIES OF RUBUS L. (ROSACEAE) IN THE ISLE OF WIGHT In 1982 two sizeable populations of Rubus effrenatus Newton, a species up till then (and still) otherwise known only in north-west Wales, v.cc. 46–49, were discovered in the Isle of Wight, v.c. 10, at a distance of 11 km from each other. One population is near the Island’s southernmost tip, mainly among bracken along a crescent of gravel overlying the chalk on the north face of Head Down but with an outlying patch in a deep ‘green lane’ about 1·4 km to the north-west. The other site is towards the Island’s south-east corner, along a much-frequented public footpath forming the north boundary of Sandown Golf Course, a relic fragment of a once-extensive tract of partly- wooded acid ground that constituted Blackpan and Lake Commons. The species is unrepresented in Rubus collections made in these two localities by 19th century specialists in the genus, and that negative evidence, taken together with a subjective impression that both populations have expanded slightly in the years since their discovery, could be interpreted as indicating a relatively recent arrival in each case (Allen 2003). Though the two may have had independent origins, it is equally possible that one population has been derived from the other – in which case that on Head Down seems the more likely to be the parent colony. In 2002–4 two successive finds of another Rubus species provided a near-duplicate of this very unexpected national distribution pattern. -

Functional Characterization of Prenyltransferases Involved in the Biosynthesis of Polycyclic Polyprenylated Acylphloroglucinols in the Genus Hypericum
Functional characterization of prenyltransferases involved in the biosynthesis of polycyclic polyprenylated acylphloroglucinols in the genus Hypericum Von der Fakultät für Lebenswissenschaften der Technischen Universität Carolo-Wilhelmina zu Braunschweig zur Erlangung des Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) genehmigte D i s s e r t a t i o n von Mohamed Mamdouh Sayed Nagia aus Kalyobiya/ Ägypten 1. Referent: Professor Dr. Ludger Beerhues 2. Referent: Professor Dr. Alain Tissier eingereicht am: 30.07.2018 mündliche Prüfung (Disputation) am: 15.10.2018 Druckjahr 2018 „Gedruckt mit Unterstützung des Deutschen Akademischen Austauschdienstes“ „Und sag: O mein Herr, mehre mein Wissen“ Der Edle Qur’an [20: 114] Vorveröffentlichungen der Dissertation Teilergebnisse aus dieser Arbeit wurden mit Genehmigung der Fakultät für Lebenswissenschaften, vertreten durch den Mentor der Arbeit, in folgenden Beiträgen vorab veröffentlicht: Publikationen Nagia, M., Gaid, M., Biedermann, E., Fiesel, T., El-Awaad, I., Haensch, R., Wittstock, U., and Beerhues, L. Sequential regiospecific gem-diprenylation of tetrahydroxyxanthone by prenyltransferases from Hypericum sp. (Submitted). Nagia, M., Gaid, M., Beuerle, T., and Beerhues, L. Successive xanthone prenylation in Hypericum sampsonii. Planta Medica International Open 4, Tu-SL-01 (2017). doi: 10.1055/s-0037-1608308 Tagungsbeiträge A. Vorträge Nagia M., Gaid M., Biedermann E., Beuerle T., Beerhues L., Successive xanthone prenylation in Hypericum sampsonii, 65th Annual Meeting of the Society for Medicinal Plant and Natural Product Research, Basel, Switzerland, 3. – 7. September 2017. Nagia M., Gaid M., Behrends S., Beerhues L., Novel PPAP-related prenyltransferases, 4. SynFoBiA -Kolloquium des Pharmaverfahrenstechnik (PVZ), Braunschweig, Germany, 26. February 2016. Nagia M., Gaid M., Beurele T., Biedermann E., Beerhues L., Aromatic Prenyltransferases from Hypericum sampsonii, Postgraduate workshop of the section „Natural Products“ German Society for Plant Sciences (DBG), Meisdorf, Germany , 11. -

Recommendation of Native Species for the Reforestation of Degraded Land Using Live Staking in Antioquia and Caldas’ Departments (Colombia)
UNIVERSITÀ DEGLI STUDI DI PADOVA Department of Land, Environment Agriculture and Forestry Second Cycle Degree (MSc) in Forest Science Recommendation of native species for the reforestation of degraded land using live staking in Antioquia and Caldas’ Departments (Colombia) Supervisor Prof. Lorenzo Marini Co-supervisor Prof. Jaime Polanía Vorenberg Submitted by Alicia Pardo Moy Student N. 1218558 2019/2020 Summary Although Colombia is one of the countries with the greatest biodiversity in the world, it has many degraded areas due to agricultural and mining practices that have been carried out in recent decades. The high Andean forests are especially vulnerable to this type of soil erosion. The corporate purpose of ‘Reforestadora El Guásimo S.A.S.’ is to use wood from its plantations, but it also follows the parameters of the Forest Stewardship Council (FSC). For this reason, it carries out reforestation activities and programs and, very particularly, it is interested in carrying out ecological restoration processes in some critical sites. The study area is located between 2000 and 2750 masl and is considered a low Andean humid forest (bmh-MB). The average annual precipitation rate is 2057 mm and the average temperature is around 11 ºC. The soil has a sandy loam texture with low pH, which limits the amount of nutrients it can absorb. FAO (2014) suggests that around 10 genera are enough for a proper restoration. After a bibliographic revision, the genera chosen were Alchornea, Billia, Ficus, Inga, Meriania, Miconia, Ocotea, Protium, Prunus, Psidium, Symplocos, Tibouchina, and Weinmannia. Two inventories from 2013 and 2019, helped to determine different biodiversity indexes to check the survival of different species and to suggest the adequate characteristics of the individuals for a successful vegetative stakes reforestation. -
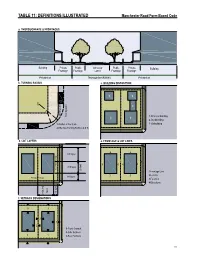
Manchester Road Redevelopment District: Form-Based Code
TaBle 11: deFiniTionS illuSTraTed manchester road Form-Based Code a. ThoroughFare & FronTageS Building Private Public Vehicular Public Private Building Frontage Frontage Lanes Frontage Frontage Private lot Thoroughfare (r.o.w.) Private lot b. Turning radiuS c. Building diSPoSiTion 3 3 2 2 1 Parking Lane Moving Lane 1- Principal Building 1 1 2- Backbuilding 1-Radius at the Curb 3- Outbuilding 2-Effective Turning Radius (± 8 ft) d. loT LAYERS e. FronTage & loT lineS 4 3rd layer 4 2 1 4 4 4 3 2nd layer Secondary Frontage 20 feet 1-Frontage Line 2-Lot Line 1st layer 3 3 Principal Frontage 3-Facades 1 1 4-Elevations layer 1st layer 2nd & 3rd & 2nd f. SeTBaCk deSignaTionS 3 3 2 1 2 1-Front Setback 2-Side Setback 1 1 3-Rear Setback 111 Manchester Road Form-Based Code ARTICLE 9. APPENDIX MATERIALS MBG Kemper Center PlantFinder About PlantFinder List of Gardens Visit Gardens Alphabetical List Common Names Search E-Mail Questions Menu Quick Links Home Page Your Plant Search Results Kemper Blog PlantFinder Please Note: The following plants all meet your search criteria. This list is not necessarily a list of recommended plants to grow, however. Please read about each PF Search Manchesterplant. Some may Road be invasive Form-Based in your area or may Code have undesirable characteristics such as above averageTab insect LEor disease 11: problems. NATIVE PLANT LIST Pests Plants of Merit Missouri Native Plant List provided by the Missouri Botanical Garden PlantFinder http://www.mobot.org/gardeninghelp/plantfinder Master Search Search limited to: Missouri Natives Search Tips Scientific Name Scientific Name Common NameCommon Name Height (ft.) ZoneZone GardeningHelp (ft.) Acer negundo box elder 30-50 2-10 Acer rubrum red maple 40-70 3-9 Acer saccharinum silver maple 50-80 3-9 Titles Acer saccharum sugar maple 40-80 3-8 Acer saccharum subsp. -

Sesbania Sesban) from the Republic of Chad: a Review
Ecology, Morphology, Distribution, and Use of Sesbania tchadica (Sesbania Sesban) from the Republic of Chad: A Review Ousman Brahim Mahamat ( [email protected] ) Abdelmalek Essaâdi University Saoud Younes Abdelmalek Essaâdi University Boy Brahim Otchom NDjamena University Steve Franzel International Centre for Research in Agroforestry: World Agroforestry Centre Al-Djazouli Ouchar Mahamat Abdelmalek Essaâdi University Asraoui Fadoua Abdelmalek Essaâdi University El ismaili Soumaya Abdelmalek Essaâdi University Systematic Review Keywords: Sesbania tchadica (Sesbania sesban), leguminosae, morphology, distribution, ora of Chad, fertilizer soil plant, medicinal plants Posted Date: May 20th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-543115/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Ecology, Morphology, Distribution, and Use of Sesbania tchadica (Sesbania Sesban) 1 from the Republic of Chad: A Review. 2 Ousman Brahim Mahamat1, Saoud Younes1 , Boy Brahim Otchom2 Steve Franzel3, Al-Djazouli Ouchar Mahamat4, Asraoui 3 Fadoua1, El ismaili Soumaya5 4 1Laboratory of Applied Biology and Pathology, Faculty of Sciences, Abdelmalek Essaâdi University, Tetouan, Morocco. 5 2Natural Substances Research Laboratory, Faculty of Exact and Applied Sciences, N‟Djamena University, Republic of Chad. 6 3International Centre for Research in Agroforestry: World Agroforestry Centre, Consultative Group on International Agricultural 7 Research, Nairobi, Kenya. 8 4Laboratory of Geology and Oceanology, Department of Geology, Faculty of Sciences, Abdelmalek Essaâdi University. 9 5Innovating Technologies Laboratory, civil engineering department, National School of Applied Sciences ENSA-Tangier, 10 Abdelmalek Essaâdi University. 11 Key message: A review of literature of potential uses and a survey study about the tree Sesbania tchadica (Sesbania Sesban) 12 leguminous native from the Republic of Chad are described in this paper. -

Biological Control of Cape Ivy Project 2004 Annual
Biological Control of Cape ivy Project 2004 Annual Research Report prepared by Joe Balciunas, Chris Mehelis, and Maxwell Chau, with contributions from Liamé van der Westhuizen and Stefan Neser Flowering Cape ivy overgrowing trees and native vegetation along California’s coast near Bolinas United States Department of Agriculture - Agricultural Research Service Western Regional Research Center - Exotic & Invasive Weed Research Unit 800 Buchanan St., Albany, California 94710 (510) 559-5975 FAX: (510) 559-5982 Executive Summary by Dr. Joe Balciunas This is our first attempt at an ‘electronic’ report, and most of you will receive our Annual Report for 2004 as PDF attachment to an email. We hope that this will make our report more easily accessible, since you may chose to store it on your hard disk. We made solid progress during 2004 towards our goal of completing our host range testing of our two most promising potential biological control agents for Cape ivy. We overcame a summertime ‘crash’ of our laboratory colonies, and by year’s end had strong colonies of both the gall fly, Parafreutreta regalis, and the stem-boring moth, Digitivalva delaireae. By the end of 2004, we had tested more than 80 species of plants, and neither of our candidate agents was able to complete development on anything other than their Cape ivy host. The single dark cloud has been the continuing downturn in external funds to support our Cape ivy research, especially that conducted by our cooperators in Pretoria, South Africa. While we have managed to maintain a small research effort there, our cooperators are no longer assisting us in our host range evaluations. -

Melilotus Alba Global Invasive Species Database (GISD)
FULL ACCOUNT FOR: Melilotus alba Melilotus alba System: Terrestrial Kingdom Phylum Class Order Family Plantae Magnoliophyta Magnoliopsida Fabales Fabaceae Common name nostrzyk bialy (Polish), white melilot (English), honey clover (English), meliloto-branco (Portuguese), hierba orejera (Spanish), donnik belyi (Russian), Weißer steinklee (German), mielga (Spanish), hubam clover (English), hubam (English), white sweetclover (English), melilot blanc (Catalan, France), Bokharaklee (German), heuin jeon dong ssa ri (Korean), bai hua cao mu xi (Chinese), hvit steinkløver (Norwegian), mielcón (Spanish), trébol de olor blanco (Spanish, Argentina, Mexico), trébol oloroso (Spanish), Vit sötväppling (Swedish), valkomesikkä (Finnish), melilot (English), white millet (English), tree clover (English), meliloto blanco (Spanish), honey-lotus (English), bokhara-clover (English, Australia, New Zealand), meliloto bianco (Italian), Shirobana shinagawa hagi (Japanese), Melilotos (Greek), Weisser honigklee (German), mélilot blanc (French), almengó blanc (Catalan, Spain), fehér somkóró (Hungarian), hvid stenkløver (Dutch) Synonym Melilotus albus , Medik Melilotus albus , var. annuus H.S. Coe Melilotus leucanthus , W.D.J. Koch ex DC. Melilotus officinalis , subsp. albus (Medik.) H. Ohashi and Tateishi Melilotus alba , Desr. Melilotus alba , L. Similar species Melilotus officinales Summary Native to Asia, Europe, and northern Africa, Melilotus alba (commonly known as white sweet clover) was introduced to the United States and first recorded in 1739. view this species on IUCN Red List Global Invasive Species Database (GISD) 2021. Species profile Melilotus alba. Pag. 1 Available from: http://www.iucngisd.org/gisd/species.php?sc=1170 [Accessed 05 October 2021] FULL ACCOUNT FOR: Melilotus alba Species Description Melilotus alba is a biennial herb with pea-like flowers attached to small stalks of elongated stems (Cole, 1990). -

Flora Da Bahia: Melastomataceae – Tibouchina S.L
DOI: 10.13102/scb1111 ARTIGO Flora da Bahia: Melastomataceae – Tibouchina s.l. Juliana Gomes Freitas1*, Andrea Karla Almeida dos Santos2,a, Paulo José Fernandes Guimarães3,b & Reyjane Patricia Oliveira1,c 1 Programa de Pós-Graduação em Botânica, Departamento de Ciências Biológicas, Universidade Estadual de Feira de Santana, Feira de Santana, Bahia, Brasil. 2 Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista, Bahia, Brasil. 3 Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Rio de Janeiro, Brasil. Resumo – É apresentado o tratamento taxonômico de Tibouchina s.l. no estado da Bahia, Brasil. São reconhecidas 37 espécies, uma delas como Pleroma (P. michelangelii). Quatro espécies são novos registros para o Nordeste (T. crassiramis, T. elegans, T. granulosa e T. sebastianopolitana), 25 são endêmicas da Bahia e nove restritas à Chapada Diamantina. O tratamento inclui chave de identificação, descrições, ilustrações, comentários e mapas de distribuição das espécies no estado. Palavras-chave adicionais: biodiversidade, Melastomeae, Pleroma, taxonomia. Abstract (Flora of Bahia: Melastomataceae – Tibouchina s.l.) – A taxonomic treatment of Tibouchina s.l. from Bahia, Brazil, is presented. Thirty-seven species are recognized, one of them in Pleroma (P. michelangelii). Four species are new records for Northeast Brazil (T. crassiramis, T. elegans, T. granulosa and T. sebastianopolitana), 25 are endemic to the state and nine restricted to the Chapada Diamantina. The treatment includes an identification key, descriptions, illustrations, comments, and distribution maps of the species in the state. Additional key words: biodiversity, Melastomeae, Pleroma, taxonomy. Melastomataceae (Myrtales) compreende cerca de não alados, glabros ou revestidos por tricomas; nós 5.000 espécies (Renner et al. -

Flora-Lab-Manual.Pdf
LabLab MManualanual ttoo tthehe Jane Mygatt Juliana Medeiros Flora of New Mexico Lab Manual to the Flora of New Mexico Jane Mygatt Juliana Medeiros University of New Mexico Herbarium Museum of Southwestern Biology MSC03 2020 1 University of New Mexico Albuquerque, NM, USA 87131-0001 October 2009 Contents page Introduction VI Acknowledgments VI Seed Plant Phylogeny 1 Timeline for the Evolution of Seed Plants 2 Non-fl owering Seed Plants 3 Order Gnetales Ephedraceae 4 Order (ungrouped) The Conifers Cupressaceae 5 Pinaceae 8 Field Trips 13 Sandia Crest 14 Las Huertas Canyon 20 Sevilleta 24 West Mesa 30 Rio Grande Bosque 34 Flowering Seed Plants- The Monocots 40 Order Alistmatales Lemnaceae 41 Order Asparagales Iridaceae 42 Orchidaceae 43 Order Commelinales Commelinaceae 45 Order Liliales Liliaceae 46 Order Poales Cyperaceae 47 Juncaceae 49 Poaceae 50 Typhaceae 53 Flowering Seed Plants- The Eudicots 54 Order (ungrouped) Nymphaeaceae 55 Order Proteales Platanaceae 56 Order Ranunculales Berberidaceae 57 Papaveraceae 58 Ranunculaceae 59 III page Core Eudicots 61 Saxifragales Crassulaceae 62 Saxifragaceae 63 Rosids Order Zygophyllales Zygophyllaceae 64 Rosid I Order Cucurbitales Cucurbitaceae 65 Order Fabales Fabaceae 66 Order Fagales Betulaceae 69 Fagaceae 70 Juglandaceae 71 Order Malpighiales Euphorbiaceae 72 Linaceae 73 Salicaceae 74 Violaceae 75 Order Rosales Elaeagnaceae 76 Rosaceae 77 Ulmaceae 81 Rosid II Order Brassicales Brassicaceae 82 Capparaceae 84 Order Geraniales Geraniaceae 85 Order Malvales Malvaceae 86 Order Myrtales Onagraceae -

Indicationes Climatices Et Geographie
PL CZ UA BRATISLAVA A H BOTANICKÁ ZÁHRADA UNIVERZITY KOMENSKÉHO Botanická 3 841 04 BRATISLAVA S L O V A K I A INDICATIONES CLIMATICES ET GEOGRAPHIE Positio geographica horti botanici: Latitudo geographica 48°09' Longitudo geographica 17°06' Altitudo super mare 145 m Indicationes climatices: (pro 51 annis 1940 – 1990) Mensibus I II III IV V VI VII VIII IX X XI XII media annua Temperatura oC -1,3 1,0 5,4 10,9 15,7 18,9 20,7 20,1 16,3 10,6 5,0 1,1 10,4 Praecipitatio mm 45 44 40 42 57 63 62 58 37 47 59 51 605 1 2 SEMINA E PLANTIS IN CALDARIIS ET IN HORTO BOTANICO CULTARUM Actinidiaceae 1 Actinidia arguta (Sieb. & Zucc.) Planch. ex Miq. 2 Actinidia chinensis Planch. var. deliciosa (A. Chev.) A. Chev. Adoxaceae 3 Sambucus caerulea Raf. 4 Viburnum burejaeticum Regel & Herd. 5 Viburnum lantana L. 6 Viburnum opulus L. subsp. calvescens (Rehd.) Sugim. 7 Viburnum rhytidophyllum Hemsl. ex Forb. & Hemsl. 8 Viburnum tinus L. Alangiaceae 9 Alangium chinense (Lour.) Harms subsp. pauciflorum W. P. Fang Alliaceae 10 * Allium angulosum L. 2.1 11 Allium cyaneum Regel 12 Allium cyathophorum Bureau & Franch. 13 Allium obliguum L. Altingiaceae 14 Liquidambar formosana Hance 15 Liquidambar orientalis Mill. Anacardiaceae 16 Rhus potaninii Maxim. 17 Toxicodendron vernicifluum (Stoke) F. A. Barkley Apiaceae 18 * Apium repens (Jacq.) Lag. 2.2 Aquifoliaceae 19 Ilex cornuta Lindl. & Paxton 20 Ilex pernyi Franch. Araliaceae 21 Aralia chinensis Blume Asparagaceae 22 Asparagus aphyllus L. 23 Danae racemosa (L.) Moench 24 Ruscus aculeatus L. -

Checkliste Der Gefäßpflanzen Irlands
Checkliste Flora Equisetum arvense ×E. Phyllitis scolopendrium = Cedrus atlantica* Salix cinerea ×S. viminalis = Ulmaceae Polygonum aviculare agg. fluviatile = Equisetum Asplenium scolopendrium Salix ×smithiana* Ulmus glabra = U. montana Polygonum aviculare Ireland ×litorale Matteuccia struthiopteris* Araucariaceae Salix cinerea ×S.purpurea Ulmus minor* = U. Polygonum maritimum Equisetum hyemale ×E. Athyrium filix-femina Araucaria araucana* xS.viminalis carpinifolia Polygonum oxyspermum systematisch variegatum = Equisetum Gymnocarpium dryopteris Salix fragilis ×S. pentandra* Ulmus procera* = U. Fagopyrum esculentum* ×trachyodon Gymnocarpium robertianum Cupressaceae Salix purpurea ×S. viminalis campestris Fallopia baldschuanica* Rot: nach CD_ROM New Cystopteris fragilis = S. ×rubra Fallopia convolvulus*# Juniperus communis Atlas of the British and Irish Polystichum aculeatum Salix triandra ×S. viminalis* Fallopia japonica* = Ophioglossaceae Juniperus communis subsp. Cannabaceae Flora; Duplikate gelöscht, Polystichum lonchitis Populus alba* Reynoutria japonica* Ophioglossum azoricum communis Cannabis sativa* wenn als autochthon Polystichum setiferum Populus nigra Fallopia sachalinensis* = Ophioglossum vulgatum Juniperus communis subsp. Humulus lupulus* nachgewiesen, dann *- Polystichum aculeatum ×P. Populus nigra (fastigiate Reynoutria sachalinensis* Botrychium lunaria nana = Juniperus Vorkommen ebenfalls setiferum = Polystichum cultivars) * Fallopia japonica ×F. communis subsp. alpina gelöscht. ×bicknellii Populus nigra subsp. Moraceae