Lee-Riding-2018.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Sponge Evolution: a Review and Phylogenetic Framework
Available online at www.sciencedirect.com ScienceDirect Palaeoworld 27 (2018) 1–29 Review Early sponge evolution: A review and phylogenetic framework a,b,∗ a Joseph P. Botting , Lucy A. Muir a Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, 39 East Beijing Road, Nanjing 210008, China b Department of Natural Sciences, Amgueddfa Cymru — National Museum Wales, Cathays Park, Cardiff CF10 3LP, UK Received 27 January 2017; received in revised form 12 May 2017; accepted 5 July 2017 Available online 13 July 2017 Abstract Sponges are one of the critical groups in understanding the early evolution of animals. Traditional views of these relationships are currently being challenged by molecular data, but the debate has so far made little use of recent palaeontological advances that provide an independent perspective on deep sponge evolution. This review summarises the available information, particularly where the fossil record reveals extinct character combinations that directly impinge on our understanding of high-level relationships and evolutionary origins. An evolutionary outline is proposed that includes the major early fossil groups, combining the fossil record with molecular phylogenetics. The key points are as follows. (1) Crown-group sponge classes are difficult to recognise in the fossil record, with the exception of demosponges, the origins of which are now becoming clear. (2) Hexactine spicules were present in the stem lineages of Hexactinellida, Demospongiae, Silicea and probably also Calcarea and Porifera; this spicule type is not diagnostic of hexactinellids in the fossil record. (3) Reticulosans form the stem lineage of Silicea, and probably also Porifera. (4) At least some early-branching groups possessed biminerallic spicules of silica (with axial filament) combined with an outer layer of calcite secreted within an organic sheath. -

On the Continuity of Background and Mass Extinction
Paleobiology, 29(4), 2003, pp. 455±467 On the continuity of background and mass extinction Steve C. Wang Abstract.ÐDo mass extinctions grade continuously into the background extinctions occurring throughout the history of life, or are they a fundamentally distinct phenomenon that cannot be explained by processes responsible for background extinction? Various criteria have been proposed for addressing this question, including approaches based on physical mechanisms, ecological se- lectivity, and statistical characterizations of extinction intensities. Here I propose a framework de®ning three types of continuity of mass and background extinc- tionsÐcontinuity of cause, continuity of effect, and continuity of magnitude. I test the third type of continuity with a statistical method based on kernel density estimation. Previous statistical ap- proaches typically have examined quantitative characteristics of mass extinctions (such as metrics of extinction intensity) and compared them with the distribution of such characteristics associated with background extinctions. If mass extinctions are outliers, or are separated by a gap from back- ground extinctions, the distinctness of mass extinctions is supported. In this paper I apply Silverman's Critical Bandwidth Test to test for the continuity of mass ex- tinctions by applying kernel density estimation and bootstrap modality testing. The method im- proves on existing work based on searching for gaps in histograms, in that it does not depend on arbitrary choices of parameters (such as bin widths for histograms), and provides a direct estimate of the signi®cance of continuities or gaps in observed extinction intensities. I am thus able to test rigorously whether differences between mass extinctions and background extinctions are statisti- cally signi®cant. -

Evolutionary Patterns of Trilobites Across the End Ordovician Mass Extinction
Evolutionary Patterns of Trilobites Across the End Ordovician Mass Extinction by Curtis R. Congreve B.S., University of Rochester, 2006 M.S., University of Kansas, 2008 Submitted to the Department of Geology and the Faculty of the Graduate School of The University of Kansas in partial fulfillment on the requirements for the degree of Doctor of Philosophy 2012 Advisory Committee: ______________________________ Bruce Lieberman, Chair ______________________________ Paul Selden ______________________________ David Fowle ______________________________ Ed Wiley ______________________________ Xingong Li Defense Date: December 12, 2012 ii The Dissertation Committee for Curtis R. Congreve certifies that this is the approved Version of the following thesis: Evolutionary Patterns of Trilobites Across the End Ordovician Mass Extinction Advisory Committee: ______________________________ Bruce Lieberman, Chair ______________________________ Paul Selden ______________________________ David Fowle ______________________________ Ed Wiley ______________________________ Xingong Li Accepted: April 18, 2013 iii Abstract: The end Ordovician mass extinction is the second largest extinction event in the history or life and it is classically interpreted as being caused by a sudden and unstable icehouse during otherwise greenhouse conditions. The extinction occurred in two pulses, with a brief rise of a recovery fauna (Hirnantia fauna) between pulses. The extinction patterns of trilobites are studied in this thesis in order to better understand selectivity of the -

Church 18.Pdf (1.785Mb)
Paleontological Contributions Number 18 Efficient Ornamentation in Ordovician Anthaspidellid Sponges Stephen B. Church August 9, 2017 Lawrence, Kansas, USA ISSN 1946-0279 (online) paleo.ku.edu/contributions Ridge-and-trough ornamented outer-wall fragment of the Ordovician anthaspidellid sponge Rugocoelia eganensis Johns, 1994. Paleontological Contributions August 9, 2017 Number 18 EFFICIENT ORNAMENTATION IN ORDOVICIAN ANTHASPIDELLID SPONGES Stephen B. Church Department of Geological Sciences, Brigham Young University, Provo, Utah 84602-3300, [email protected] ABSTRACT Lithistid orchoclad sponges within the family Anthaspidellidae Ulrich in Miller, 1889 include several genera that added ornate features to their outer-wall surfaces during Early Ordovician sponge radiation. Ornamented anthaspidellid sponges commonly constructed annulated or irregularly to regularly spaced transverse ridge-and-trough features on their outer-wall surfaces without proportionately increasing the size of their internal wall or gastral surfaces. This efficient technique of modi- fying only the sponge’s outer surface without enlarging its entire skeletal frame conserved the sponge’s constructional energy while increasing outer-wall surface-to-fluid exposure for greater intake of nutrient bearing currents. Sponges with widely spaced ridge-and-trough ornament dimensions predominated in high-energy settings. Widely spaced ridges and troughs may have given the sponge hydrodynamic benefits in high wave force settings. Ornamented sponges with narrowly spaced ridge-and- trough dimensions are found in high energy paleoenvironments but also occupied moderate to low-energy settings, where their surface-to-fluid exposure per unit area exceeded that of sponges with widely spaced surface ornamentations. Keywords: lithistid sponges, Ordovician radiation, morphological variation, theoretical morphology INTRODUCTION assumed by most anthaspidellids. -

Stromatoporoids in the Devonian Carbonate Complex in Moravia (Czechoslovakia)
ACT A POLONICA Vol. 25 No. 3-4 VLASTA ZUKALOVA STROMATOPOROIDS IN THE DEVONIAN CARBONATE COMPLEX IN MORAVIA (CZECHOSLOVAKIA) ZUKALOVA, v.: Stromatoporoids in the Devonian carbonate complex in Mo ravia (Czechoslovakia). Acta Palaeont. Polonica, 25, 3/4, 671-679, December 1981. Studies of the Paleozoic rocks in Moravia based on abundant drillings reveal the extent of the Devonian reefs (s.!.) beneath the Carpathia~ Flysh Belt and Neogene foredeeps. Reef limestones (rich mainly in stromatoporoids) are re stricted to the platform part of the sedimentary basin. A 'gradual transgression reached this area during the Givetian and Frasnian having its culmination in the Early Frasnian. Development of reef limestones in Moravia ceased at the Frasnian/Famennian boundary. Key W 0 r d s: Stromatoporoidea, stratigraphy, Devonian, Czechoslovakia. Vlasta Zukalova. Ostfedni Ostav Geo!ogicIQ/, 60200 Brno, Leitnerova 22, Cze choslovakia. Received: September 1979. The Paleozoic' sedimentary basin is bounded on the west by meta morphosed crystalline rocks. Paleozoic deposits are covered by the Carpa thian nappes and the Neogene foredeep fillings on the south and south east, while they extend into Polish territory on the north and northeast. The Silurian graptolite shales occurring near the village of Stinava are the most ancient sediments for which there is paleontological evidence in Moravia (Bollcek 1935). They give evidence of the earliest marine trans gression over Moravia. During Paleozoic time, deep sea conditions pre vailed in this area where mainly shales with subordinate limestone inter calations were deposited. The Lower Devonian (Siegenian) transgression took place over a re stricted area. Relics of fauna in the quartzites are known at the villages of Zlate Horyand Vrbno. -
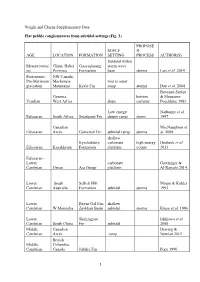
Wright and Cherns Supplementary Data Flat
Wright and Cherns Supplementary Data Flat pebble conglomerates from subtidal settings (Fig. 2) PROPOSE SHELF D AGE LOCATION FORMATION SETTING PROCESS AUTHOR(S) Subtidal within Mesoproteroz China, Hebei Gaoyuzhuang storm wave oic Province Formation base storms Luo et al. 2014 Proterozoic, NW Canada, Pre-Marinoan Mackenzie mid to outer glaciation Mountains Keele Fm ramp storms Day et al. 2004 Bertrand-Sarfati Gourma, bottom & Moussine- Vendian West Africa slope currents' Pouchkine 1983 Low energy Narbonne et al. Ediacaran South Africa Swartpunt Fm deeper ramp storm 1997 Canadian MacNaughton et Ediacaran Arctic Gametrail Fm subtidal ramp storms al. 2008 shallow Kyrshabakty carbonate high energy Heubeck et al. Ediacaran Kazakhstan Formation platform events 2013 Ediacaran - Lower carbonate Grotzinger & Cambrian Oman Ara Group platform Al-Rawahi 2014 Lower South Sellick Hill Mount & Kidder Cambrian Australia Formation subtidal storms 1993 Lower Bayan Gol Fm, shallow Cambrian W Mongolia Zavkhan Basin subtidal storms Kruse et al. 1996 Lower Shuijingtuo Ishikawa et al. Cambrian South China Fm subtidal 2008 Middle Canadian Dewing & Cambrian Arctic ramp Nowlan 2012 British Middle Columbia, Cambrian Canada Jubilee Fm Pope 1990 1 Middle Pratt & Cambrian Argentina La Laja Fm subtidal shelf tsunamis Bordonaro 2007 low energy Middle shallow Cambrian Australia Ranken Lst subtidal storms Kruse 1996 Middle Wyoming, Upper Gros Cambrian USA Ventre Shale Csonka 2009 middle upper Middle W Utah, upper Wheeler, carbonate belt - Cambrian USA Marjum fms subtidal shelf Robison 1964 Middle- Upper Supratidal to Cambrian NW China subtidal fpc storms Liang et al. 1993 Ust’- Brus,Labaz, Middle Orakta, Cambrian - Kulyumbe, Lower Ujgur and Iltyk Kouchinsky et Ordovician Siberia fms al. -

Multiscale Approach Reveals That Cloudina Aggregates Are Detritus
Multiscale approach reveals that Cloudina aggregates PNAS PLUS are detritus and not in situ reef constructions Akshay Mehraa,1 and Adam Maloofa aDepartment of Geosciences, Princeton University, Princeton, NJ 08544 Edited by Donald E. Canfield, Institute of Biology and Nordic Center for Earth Evolution, University of Southern Denmark, Odense M., Denmark, and approved January 19, 2018 (received for review November 14, 2017) The earliest metazoans capable of biomineralization appeared precluding physical separation or the use of traditional computed during the late Ediacaran Period (635–541 Ma) in strata associ- tomography (CT) techniques. The inability to produce in situ ated with shallow water microbial reefs. It has been suggested 3D reconstructions has led researchers to make measurements that some Ediacaran microbial reefs were dominated (and possi- of Cloudina individuals and aggregates on polished slabs, thin bly built) by an abundant and globally distributed tubular organ- sections, and bedding planes (3, 4, 7). Unfortunately, as noted ism known as Cloudina. If true, this interpretation implies that by previous researchers, 3D spatial and size distributions can- metazoan framework reef building—a complex behavior that is not be estimated from 2D cross-sections (12). Furthermore, syn- responsible for some of the largest bioconstructions and most thetic experiments reveal that, in the case of tubular structures diverse environments in modern oceans—emerged much earlier such as Cloudina, it is not possible to correctly infer orienta- than previously thought. Here, we present 3D reconstructions of tion from 2D cross-sections (Fig. 1 A–C), and diameter measure- Cloudina populations, produced using an automated serial grind- ments made on cross-sections through curved and/or elliptical ing and imaging system coupled with a recently developed neural tubes are subject to a large degree of error (as great as 35%; Fig. -

Appendix 3.Pdf
A Geoconservation perspective on the trace fossil record associated with the end – Ordovician mass extinction and glaciation in the Welsh Basin Item Type Thesis or dissertation Authors Nicholls, Keith H. Citation Nicholls, K. (2019). A Geoconservation perspective on the trace fossil record associated with the end – Ordovician mass extinction and glaciation in the Welsh Basin. (Doctoral dissertation). University of Chester, United Kingdom. Publisher University of Chester Rights Attribution-NonCommercial-NoDerivatives 4.0 International Download date 26/09/2021 02:37:15 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10034/622234 International Chronostratigraphic Chart v2013/01 Erathem / Era System / Period Quaternary Neogene C e n o z o i c Paleogene Cretaceous M e s o z o i c Jurassic M e s o z o i c Jurassic Triassic Permian Carboniferous P a l Devonian e o z o i c P a l Devonian e o z o i c Silurian Ordovician s a n u a F y r Cambrian a n o i t u l o v E s ' i k s w o Ichnogeneric Diversity k p e 0 10 20 30 40 50 60 70 S 1 3 5 7 9 11 13 15 17 19 21 n 23 r e 25 d 27 o 29 M 31 33 35 37 39 T 41 43 i 45 47 m 49 e 51 53 55 57 59 61 63 65 67 69 71 73 75 77 79 81 83 85 87 89 91 93 Number of Ichnogenera (Treatise Part W) Ichnogeneric Diversity 0 10 20 30 40 50 60 70 1 3 5 7 9 11 13 15 17 19 21 n 23 r e 25 d 27 o 29 M 31 33 35 37 39 T 41 43 i 45 47 m 49 e 51 53 55 57 59 61 c i o 63 z 65 o e 67 a l 69 a 71 P 73 75 77 79 81 83 n 85 a i r 87 b 89 m 91 a 93 C Number of Ichnogenera (Treatise Part W) -

Review of the Mineralogy of Calcifying Sponges
Dickinson College Dickinson Scholar Faculty and Staff Publications By Year Faculty and Staff Publications 12-2013 Not All Sponges Will Thrive in a High-CO2 Ocean: Review of the Mineralogy of Calcifying Sponges Abigail M. Smith Jade Berman Marcus M. Key, Jr. Dickinson College David J. Winter Follow this and additional works at: https://scholar.dickinson.edu/faculty_publications Part of the Paleontology Commons Recommended Citation Smith, Abigail M.; Berman, Jade; Key,, Marcus M. Jr.; and Winter, David J., "Not All Sponges Will Thrive in a High-CO2 Ocean: Review of the Mineralogy of Calcifying Sponges" (2013). Dickinson College Faculty Publications. Paper 338. https://scholar.dickinson.edu/faculty_publications/338 This article is brought to you for free and open access by Dickinson Scholar. It has been accepted for inclusion by an authorized administrator. For more information, please contact [email protected]. © 2013. Licensed under the Creative Commons http://creativecommons.org/licenses/by- nc-nd/4.0/ Elsevier Editorial System(tm) for Palaeogeography, Palaeoclimatology, Palaeoecology Manuscript Draft Manuscript Number: PALAEO7348R1 Title: Not all sponges will thrive in a high-CO2 ocean: Review of the mineralogy of calcifying sponges Article Type: Research Paper Keywords: sponges; Porifera; ocean acidification; calcite; aragonite; skeletal biomineralogy Corresponding Author: Dr. Abigail M Smith, PhD Corresponding Author's Institution: University of Otago First Author: Abigail M Smith, PhD Order of Authors: Abigail M Smith, PhD; Jade Berman, PhD; Marcus M Key Jr, PhD; David J Winter, PhD Abstract: Most marine sponges precipitate silicate skeletal elements, and it has been predicted that they would be among the few "winners" in an acidifying, high-CO2 ocean. -

Rowan C. Martindale Curriculum Vitae Associate Professor (Invertebrate Paleontology) at the University of Texas at Austin
ROWAN C. MARTINDALE CURRICULUM VITAE ASSOCIATE PROFESSOR (INVERTEBRATE PALEONTOLOGY) AT THE UNIVERSITY OF TEXAS AT AUSTIN Department of Geological Sciences E-mail: [email protected] Jackson School of Geosciences Website: www.jsg.utexas.edu/martindale/ 2275 Speedway Stop C9000 Orchid ID: 0000-0003-2681-083X Austin, TX 78712-1722 Phone: 512-475-6439 Office: JSG 3.216A RESEARCH INTERESTS The overarching theme of my work is the connection between Earth and life through time, more precisely, understanding ancient (Mesozoic and Cenozoic) ocean ecosystems and the evolutionary and environmental events that shaped them. My research is interdisciplinary, (paleontology, sedimentology, biology, geochemistry, and oceanography) and focuses on: extinctions and carbon cycle perturbation events (e.g., Oceanic Anoxic Events, acidification events); marine (paleo)ecology and reef systems; the evolution of reef builders (e.g., coral photosymbiosis); and exceptionally preserved fossil deposits (Lagerstätten). ACADEMIC APPOINTMENTS Associate Professor, University of Texas at Austin September 2020 to Present Assistant Professor, University of Texas at Austin August 2014 to August 2020 Postdoctoral Researcher, Harvard University August 2012 to July 2014 Department of Organismic and Evolutionary Biology; Mentor: Dr. Andrew H. Knoll. EDUCATION Doctorate, University of Southern California 2007 to 2012 Dissertation: “Paleoecology of Upper Triassic reef ecosystems and their demise at the Triassic-Jurassic extinction, a potential ocean acidification event”. Advisor: Dr. David J. Bottjer, degree conferred August 7th, 2012. Bachelor of Science Honors Degree, Queen’s University 2003 to 2007 Geology major with a general concentration in Biology (Geological Sciences Medal Winner). AWARDS AND RECOGNITION Awards During Tenure at UT Austin • 2019 National Science Foundation CAREER Award: Awarded to candidates who are judged to have the potential to serve as academic role models in research and education. -

Western North Greenland (Laurentia)
BULLETIN OF THE GEOLOGICAL SOCIETY OF DENMARK · VOL. 69 · 2021 Trilobite fauna of the Telt Bugt Formation (Cambrian Series 2–Miaolingian Series), western North Greenland (Laurentia) JOHN S. PEEL Peel, J.S. 2021. Trilobite fauna of the Telt Bugt Formation (Cambrian Series 2–Mi- aolingian Series), western North Greenland (Laurentia). Bulletin of the Geological Society of Denmark, Vol. 69, pp. 1–33. ISSN 2245-7070. https://doi.org/10.37570/bgsd-2021-69-01 Trilobites dominantly of middle Cambrian (Miaolingian Series, Wuliuan Stage) Geological Society of Denmark age are described from the Telt Bugt Formation of Daugaard-Jensen Land, western https://2dgf.dk North Greenland (Laurentia), which is a correlative of the Cape Wood Formation of Inglefield Land and Ellesmere Island, Nunavut. Four biozones are recognised in Received 6 July 2020 Daugaard-Jensen Land, representing the Delamaran and Topazan regional stages Accepted in revised form of the western USA. The basal Plagiura–Poliella Biozone, with Mexicella cf. robusta, 16 December 2020 Kochiella, Fieldaspis? and Plagiura?, straddles the Cambrian Series 2–Miaolingian Series Published online 20 January 2021 boundary. It is overlain by the Mexicella mexicana Biozone, recognised for the first time in Greenland, with rare specimens of Caborcella arrojosensis. The Glossopleura walcotti © 2021 the authors. Re-use of material is Biozone, with Glossopleura, Clavaspidella and Polypleuraspis, dominates the succes- permitted, provided this work is cited. sion in eastern Daugaard-Jensen Land but is seemingly not represented in the type Creative Commons License CC BY: section in western outcrops, likely reflecting the drastic thinning of the formation https://creativecommons.org/licenses/by/4.0/ towards the north-west. -

Extinction Events Among Jurassic Bivalves
中国科技论文在线 http://www.paper.edu.cn 中山大学学报 ( 自然科学版) 第 39 卷 第 1 期 ACTA SCIENTIARUM NATURALIUM Vol 39 No1 2000 年 1 月 UNIVERSITATIS SUNYATSENI Jan 2000 Article ID: 05296579 ( 2000) 01009105 Extinction Events Among Jurassic Bivalves LIU Chunlian ( Department of Earth Sciences, Zhongshan University, Guangzhou 510275) Abstract: Generic/ subgeneric level data on bivalves from the Jurassic ProtoAtlantic record three regional extinction events, at the end of the Pliensbachian, beginning of the Callovian and Tithonian stages. The ex tinction at the T ithonian is the most important in terms of magnitude and duration. These extinctions can cor relate with sealevel changes and associated environmental deterioration. The endPliensbachian extinction, related to anoxia caused by a sharp rise of sea level, selectively eliminated infaunal bivalves. In the Callo vian event, which was linked to a regional regression, the selection against infaunal group occurred only in midlatitude area. T ithonian event was a result of extreme and prolonged regression and lacked the selective extinction of infaunal bivalves. Keywords: extinction; Jurassic bivalves; ProtoAtlantic CLC number: Q9158174 Document code: A 1 Introduction Two extinction events among Jurassic organisms, at the end of the Pliensbachian and Tithonian stages, were confirmed at family level by Sepkoski et al[ 1] . Using specieslevel data of the molluscs, Hallam[ 2] demostrated that marine invertebrate mass extinctions at these times occurred on a regional, not a global scale. He estimated that 84% of species became extinct in West Europe in the endPliens bachian extinction, which was considered the most important of the whole Jurassic. However, no de tailed studies on the Jurassic extinctions at the generic level were published up to date.