Global Warming and Biodiversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Towards an Understanding of the Evolution of Violaceae from an Anatomical and Morphological Perspective Saul Ernesto Hoyos University of Missouri-St
University of Missouri, St. Louis IRL @ UMSL Theses Graduate Works 8-7-2011 Towards an understanding of the evolution of Violaceae from an anatomical and morphological perspective Saul Ernesto Hoyos University of Missouri-St. Louis, [email protected] Follow this and additional works at: http://irl.umsl.edu/thesis Recommended Citation Hoyos, Saul Ernesto, "Towards an understanding of the evolution of Violaceae from an anatomical and morphological perspective" (2011). Theses. 50. http://irl.umsl.edu/thesis/50 This Thesis is brought to you for free and open access by the Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Theses by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. Saul E. Hoyos Gomez MSc. Ecology, Evolution and Systematics, University of Missouri-Saint Louis, 2011 Thesis Submitted to The Graduate School at the University of Missouri – St. Louis in partial fulfillment of the requirements for the degree Master of Science July 2011 Advisory Committee Peter Stevens, Ph.D. Chairperson Peter Jorgensen, Ph.D. Richard Keating, Ph.D. TOWARDS AN UNDERSTANDING OF THE BASAL EVOLUTION OF VIOLACEAE FROM AN ANATOMICAL AND MORPHOLOGICAL PERSPECTIVE Saul Hoyos Introduction The violet family, Violaceae, are predominantly tropical and contains 23 genera and upwards of 900 species (Feng 2005, Tukuoka 2008, Wahlert and Ballard 2010 in press). The family is monophyletic (Feng 2005, Tukuoka 2008, Wahlert & Ballard 2010 in press), even though phylogenetic relationships within Violaceae are still unclear (Feng 2005, Tukuoka 2008). The family embrace a great diversity of vegetative and floral morphologies. Members are herbs, lianas or trees, with flowers ranging from strongly spurred to unspurred. -

Growing Alcantarea
Bromeliaceae VOLUME XLII - No. 3 - MAY/JUNE 2008 The Bromeliad Society of Queensland Inc. P. O. Box 565, Fortitude Valley Queensland, Australia 4006, Home Page www.bromsqueensland.com OFFICERS PRESIDENT Olive Trevor (07) 3351 1203 VICE PRESIDENT Anne McBurnie PAST PRESIDENT Bob Reilly (07) 3870 8029 SECRETARY Chris Coulthard TREASURER Glenn Bernoth (07) 4661 3 634 BROMELIACEAE EDITOR Ross Stenhouse SHOW ORGANISER Bob Cross COMMITTEE Greg Aizlewood, Bruce Dunstan, Barry Kable, Arnold James,Viv Duncan, David Rees MEMBERSHIP SECRETARY Roy Pugh (07) 3263 5057 SEED BANK CO-ORDINATOR Doug Parkinson (07) 5497 5220 AUDITOR Anna Harris Accounting Services SALES AREA CASHIER Norma Poole FIELD DAY CO-ORDINATOR Ruth Kimber & Bev Mulcahy LIBRARIAN Evelyn Rees ASSISTANT SHOW ORGANISER Phil Beard SUPPER STEWARDS Nev Ryan, Barry Genn PLANT SALES Pat Barlow Phil James COMPETITION STEWARDS Dorothy Cutcliffe, Arnold James CHIEF COMPETITION STEWARD HOSTESS Gwen Parkinson BSQ WEBMASTER Ross Stenhouse LIFE MEMBERS Grace Goode OAM Peter Paroz, Michael O’Dea Editors Email Address: [email protected] The Bromeliad Society of Queensland Inc. gives permission to all Bromeliad Societies to re- print articles in their journals provided proper acknowledgement is given to the original author and the Bromeliaceae, and no contrary direction is published in Bromeliaceae. This permission does not apply to any other person or organisation without the prior permission of the author. Opinions expressed in this publication are those of the individual contributor and may not neces- sarily reflect the opinions of the Bromeliad Society of Queensland or of the Editor Authors are responsible for the accuracy of the information in their articles. -
Ber Gl an D V on G U a Y A
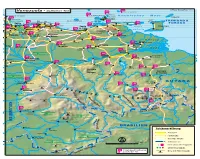
ISLAS LOS ROQUES ©REISE KNOW-HOW 2012 Venezuela - östlicher Teil 629 622 ISLAS LOS TESTIGOS 586 ISLAS LOS FRAILES San Juan de los Cayos ISLA MARGARITA Karibisches Meer 578 La Asunción TOBAGO Chichiriviche Porlamar Punta 268 PORT TRINIDAD & o Nationalpark 209 La Los ISLA LA TORTUGA de Piedras OF SPAIN Morrocoy GuairaCaracas Carúpano 289 Puerto Maiquetía 216 245 TOBAGO Morón Choroní Chuspa Irapa Güiria Cabello 169 Higuerote Cumaná Cariaco Casanay 558 537 Guatire Puerto Golfo TRINIDAD Henri Maracay CARACAS Caucagua Cueva del Valencia Pittier 226 La Cruz Cagua El Guapo Guácharo deParia San Fernando Nat. Park Cua Barcelona San Caripito a Bejuma Francisco Villa de 431 Cura Santa Fé La Encrucijada La Toscana 299 Tinaquillo San Juan Altagracia de los Morros Maturín Tinaco de Orituco Aragua de Barcelona 316 Anaco Orinoco- Chaguaramas La Horqueta El Sombrero Zaraza 306 Los Güires 436 Tucupita 434 Valle de El Tigrito Temblador El Baúl la Pascua 317 San Antonio Calabozo El Tigre de Tabasca Delta Moja 322 Barrancas Casabe Ciudad Nationalpark Guayana 333 Aguaro-Guariquito Río Gua Ciudad 361 Campamento na r 416 Río Grande e Bolívar Upata San Fernando Apurito Moitaco El Palmar de Apure Mapire Achaguas Cabruta 398 R Caicara 345 ío A Bochinche pure del Orinoco Las Ciudad ual o Adjuntas 363 c Piar Embalse 363 o n de Guri ri El Callao Tumeremo O El Manteco Río Serranía La Urbana Serranía Turagua 345 de Imataca La Vergareña Paragua 364 Nationalpark Guaniamo El Dorado Santos Luzardo 410 Puerto Río ío Meta C R a Páez u GUYANA Puerto r San Isidro a 346 Carreño -

Network Scan Data
Selbyana 15: 132-149 CHECKLIST OF VENEZUELAN BROMELIACEAE WITH NOTES ON SPECIES DISTRIBUTION BY STATE AND LEVELS OF ENDEMISM BRUCE K. HOLST Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, USA ABSTRACf. A checklist of the 24 genera and 364 native species ofBromeliaceae known from Venezuela is presented, including their occurrence by state and indications of which are endemic to the country. A comparison of the number of genera and species known from Mesoamerica (southern Mexico to Panama), Colombia, Venezuela, the Guianas (Guyana, Suriname, French Guiana), Ecuador, and Peru is presented, as well as a summary of the number of species and endemic species in each Venezuelan state. RESUMEN. Se presenta un listado de los 24 generos y 364 especies nativas de Bromeliaceae que se conocen de Venezuela, junto con sus distribuciones por estado y una indicaci6n cuales son endemicas a Venezuela. Se presenta tambien una comparaci6n del numero de los generos y especies de Mesoamerica (sur de Mexico a Panama), Colombia, Venezuela, las Guayanas (Guyana, Suriname, Guyana Francesa), Ecuador, y Peru, y un resumen del numero de especies y numero de especies endemicas de cada estado de Venezuela. INTRODUCTION Bromeliaceae (Smith 1971), and Revision of the Guayana Highland Bromeliaceae (Smith 1986). The checklist ofVenezuelan Bromeliaceae pre Several additional country records were reported sented below (Appendix 1) adds three genera in works by Smith and Read (1982), Luther (Brewcaria, Neoregelia, and Steyerbromelia) and (1984), Morillo (1986), and Oliva-Esteva and 71 species to the totals for the country since the Steyermark (1987). Author abbreviations used last summary of Venezuelan bromeliads in the in the checklist follow Brummit and Powell Flora de Venezuela series which contained 293 (1992). -

Alphabetical Lists of the Vascular Plant Families with Their Phylogenetic
Colligo 2 (1) : 3-10 BOTANIQUE Alphabetical lists of the vascular plant families with their phylogenetic classification numbers Listes alphabétiques des familles de plantes vasculaires avec leurs numéros de classement phylogénétique FRÉDÉRIC DANET* *Mairie de Lyon, Espaces verts, Jardin botanique, Herbier, 69205 Lyon cedex 01, France - [email protected] Citation : Danet F., 2019. Alphabetical lists of the vascular plant families with their phylogenetic classification numbers. Colligo, 2(1) : 3- 10. https://perma.cc/2WFD-A2A7 KEY-WORDS Angiosperms family arrangement Summary: This paper provides, for herbarium cura- Gymnosperms Classification tors, the alphabetical lists of the recognized families Pteridophytes APG system in pteridophytes, gymnosperms and angiosperms Ferns PPG system with their phylogenetic classification numbers. Lycophytes phylogeny Herbarium MOTS-CLÉS Angiospermes rangement des familles Résumé : Cet article produit, pour les conservateurs Gymnospermes Classification d’herbier, les listes alphabétiques des familles recon- Ptéridophytes système APG nues pour les ptéridophytes, les gymnospermes et Fougères système PPG les angiospermes avec leurs numéros de classement Lycophytes phylogénie phylogénétique. Herbier Introduction These alphabetical lists have been established for the systems of A.-L de Jussieu, A.-P. de Can- The organization of herbarium collections con- dolle, Bentham & Hooker, etc. that are still used sists in arranging the specimens logically to in the management of historical herbaria find and reclassify them easily in the appro- whose original classification is voluntarily pre- priate storage units. In the vascular plant col- served. lections, commonly used methods are systema- Recent classification systems based on molecu- tic classification, alphabetical classification, or lar phylogenies have developed, and herbaria combinations of both. -

17 Tribo Gnaphalieae (Cass.) Lecoq
17 Tribo Gnaphalieae (Cass.) Lecoq. & Juill. Leonardo Paz Deble SciELO Books / SciELO Livros / SciELO Libros DEBLE, L.P. Tribo Gnaphalieae (Cass.) Lecoq. & Juill. In: ROQUE, N. TELES, A.M., and NAKAJIMA, J.N., comp. A família Asteraceae no Brasil: classificação e diversidade [online]. Salvador: EDUFBA, 2017, pp. 131-137. ISBN: 978-85-232-1999-4. https://doi.org/10.7476/9788523219994.0019. All the contents of this work, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 International license. Todo o conteúdo deste trabalho, exceto quando houver ressalva, é publicado sob a licença Creative Commons Atribição 4.0. Todo el contenido de esta obra, excepto donde se indique lo contrario, está bajo licencia de la licencia Creative Commons Reconocimento 4.0. 17 TRIBO GNAPHALIEAE (CASS.) LECOQ. & JUILL. Leonardo Paz Deble A tribo Gnaphalieae é composta de 180-190 gêneros e cerca de 1.240 espécies, com distribuição quase cosmopolita, com maior diversidade na África do Sul, Ásia, Austrália e América do Sul (BAYER et al., 2007; DILLON; SAGASTEGUI, 1991; WARD et al., 2009). Para a América do Sul, Dillon e Sagastegui (1991) reconheceram 2 centros de diversidade: a Cordilheira dos Andes e o Sudeste do Brasil e regiões adjacentes. Tradicionalmente, Gnaphalieae foi subordinada à tribo Inuleae, sen- do reconhecida como um dos grupos de mais difícil identificação dentro da família, dadas a homogeneidade dos caracteres vegetativos e a escassa variação dos caracteres florais (CABRERA, 1961). Estudos recentes demons- traram que as Gnaphalieae encerram grupo monofilético posicionado na base da subfamília Asteroideae (ANDERBERG, 2009; ANDERBERG et al., 2005; FUNK et al., 2005). -

Pandon Ekamanin.Pdf
© Texto e ilustraciones: Hanneke Wagenaar © Fundación Editorial El perro y la rana, 2010 Centro Simón Bolívar Torre Norte, piso 21, El Silencio, Caracas - Venezuela, 1010. Teléfonos: (0212) 7688300 / 7688399. Correos electrónicos: [email protected] [email protected] Páginas web: www.elperroylarana.gob.ve www.ministeriodelacultura.gob.ve Edición al cuidado de: Rodolfo Castillo Elis Labrador Mónica Piscitelli Hecho el Depósito de Ley Depósito legal lf 40220108002277 ISBN 978-980-14-1076-8 IMPRESO EN VENEZUELA Hanneke Wagenaar Pandón ekamanín (Cuentacuentos) Al valle de Kamarata Nota de la ekamanín La primera idea que me asalta es que no son mis cuentos, son de los pemón, de su tradición oral, a ellos les pertenecen. La segunda idea es que, de ninguna manera, este trabajo tiene pretensiones antro- pológicas. Son sencillamente la razón para dibujar tepuyes, selvas y sabanas; arcoíris, nubes y cielos; personas que son pájaros y árboles que son personas; rocas parlantes, tucusitos en busca de una flor y acures que viajan dentro de troncos huecos en un mundo alucinado; anacondas gigantes y cerros que se abren para dibujar una tierra que me ha impactado quedándose pegada al cuerpo como una nueva capa de piel, utilizando los colores del cuero sutil de la boa tornasolada con la que los pájaros se cubrie- ron en el tiempo de Piá, cuando todo lo nombrable era humano. Es por ello -y con todo mi respeto- que escribo, a mi entender, los cuentos que escuché o leí en algún momento y que ahora les cuento a los karán, es decir, a los visitantes de otras latitudes y a los tüponkén de la ciudad. -

12. Plantas Y Líquenes Del Parque Nacional Natural De La Serranía De
PLANTAS Y LÍQUENES DEL PARQUE NACIONAL NATURAL SERRANÍA DE CHIRIBIQUETE, COLOMBIA Dairon Cárdenas1*1*,, MaríaMaría FernandaFernanda GonzálezGonzález12 ,, NóridaNórida MarínMarín11 y Sonia Sua11,, JulioJulio BetancurBetancur2 RESUMEN principales de formaciones vegetales registrados para la Guayana: bosques, matorrales, praderas o Se presenta el inventario de las plantas y los pastizales y la vegetación pionera sobre roca dura. líquenes del Parque Nacional Natural Serranía de En los afloramientos rocosos predominan especies Chiribiquete (PNNSCh), obtenido a través de como: Abolboda spp., Acanthella sprucei, Bonnetia información de herbarios y exploraciones de campo. sessilis, Calliandra vaupesiana, Clusia spp., Cyrilla Chiribiquete es el área protegida más grande de racemiflora, Decagonocarpus oppositifolius, Drosera Colombia, pertenece a la formación Guayana, esmeraldae, Euphronia hirtelloides, Gongylolepis tiene altitudes entre 200 y 800 metros y representa una gran meseta tectónica fraccionada por erosión martiana, Hevea nitida, Molongum lucidum, hídrica y disectada en numerosas mesas (tepuyes). Navia spp., Ochthocosmus berryi, Monotrema aemu- Se registraron 2,138 especies que corresponden lans, Pachira coriacea, Parahancornia surrogata, al 7.6 % de las encontradas en Colombia y al 66.5 Senefelderopsis chiribiquetensis, Steyerbromelia % de las de la región amazónica. Las familias con garcia-barrigae, Styrax rigidifolius, Tepuianthus más especies fueron: Fabaceae (184), Rubiaceae savannensis, Utricularia spp., Vellozia tubiflora (167) -

Evolutionary History of Floral Key Innovations in Angiosperms Elisabeth Reyes
Evolutionary history of floral key innovations in angiosperms Elisabeth Reyes To cite this version: Elisabeth Reyes. Evolutionary history of floral key innovations in angiosperms. Botanics. Université Paris Saclay (COmUE), 2016. English. NNT : 2016SACLS489. tel-01443353 HAL Id: tel-01443353 https://tel.archives-ouvertes.fr/tel-01443353 Submitted on 23 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NNT : 2016SACLS489 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de Doctorat : Biologie Par Mme Elisabeth Reyes Evolutionary history of floral key innovations in angiosperms Thèse présentée et soutenue à Orsay, le 13 décembre 2016 : Composition du Jury : M. Ronse de Craene, Louis Directeur de recherche aux Jardins Rapporteur Botaniques Royaux d’Édimbourg M. Forest, Félix Directeur de recherche aux Jardins Rapporteur Botaniques Royaux de Kew Mme. Damerval, Catherine Directrice de recherche au Moulon Président du jury M. Lowry, Porter Curateur en chef aux Jardins Examinateur Botaniques du Missouri M. Haevermans, Thomas Maître de conférences au MNHN Examinateur Mme. Nadot, Sophie Professeur à l’Université Paris-Sud Directeur de thèse M. -

Gnaphalieae-Asteraceae) of Mexico
Botanical Sciences 92 (4): 489-491, 2014 TAXONOMY AND FLORISTIC NEW COMBINATIONS IN PSEUDOGNAPHALIUM (GNAPHALIEAE-ASTERACEAE) OF MEXICO OSCAR HINOJOSA-ESPINOSA Y JOSÉ LUIS VILLASEÑOR1 Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México, México, D.F., México 1Corresponding author: [email protected] Abstract: In a broad sense, Gnaphalium L. is a heterogeneous and polyphyletic genus. Pseudognaphalium Kirp. is one of the many segregated genera from Gnaphalium which have been proposed to obtain subgroups that are better defi ned and presumably monophyletic. Although most Mexican species of Gnaphalium s.l. have been transferred to Pseudognaphalium, the combinations so far proposed do not include a few Mexican taxa that truly belong in Pseudognaphalium. In this paper, the differences between Gnaphalium s.s. and Pseudognaphalium are briefl y addressed, and the transfer of two Mexican species and three varieties from Gnaphalium to Pseudognaphalium are presented. Key Words: generic segregate, Gnaphalium, Mexican composites, taxonomy. Resumen: En sentido amplio, Gnaphalium L. es un género heterogéneo y polifi lético. Pseudognaphalium Kirp. es uno de varios géneros segregados, a partir de Gnaphalium, que se han propuesto para obtener subgrupos mejor defi nidos y presumiblemente monofi léticos. La mayoría de las especies mexicanas de Gnaphalium s.l. han sido transferidas al género Pseudognaphalium; sin embargo, las combinaciones propuestas hasta el momento no cubren algunos taxones mexicanos que pertenecen a Pseudogna- phalium. En este trabajo se explican brevemente las diferencias entre Gnaphalium s.s. y Pseudognaphalium, y se presentan las transferencias de dos especies y tres variedades mexicanas de Gnaphalium a Pseudognaphalium. Palabras clave: compuestas mexicanas, Gnaphalium, segregados genéricos, taxonomía. -

Taxonomic Revision of the Chilean Puya Species (Puyoideae
Taxonomic revision of the Chilean Puya species (Puyoideae, Bromeliaceae), with special notes on the Puya alpestris-Puya berteroniana species complex Author(s): Georg Zizka, Julio V. Schneider, Katharina Schulte and Patricio Novoa Source: Brittonia , 1 December 2013, Vol. 65, No. 4 (1 December 2013), pp. 387-407 Published by: Springer on behalf of the New York Botanical Garden Press Stable URL: https://www.jstor.org/stable/24692658 JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at https://about.jstor.org/terms New York Botanical Garden Press and Springer are collaborating with JSTOR to digitize, preserve and extend access to Brittonia This content downloaded from 146.244.165.8 on Sun, 13 Dec 2020 04:26:58 UTC All use subject to https://about.jstor.org/terms Taxonomic revision of the Chilean Puya species (Puyoideae, Bromeliaceae), with special notes on the Puya alpestris-Puya berteroniana species complex Georg Zizka1'2, Julio V. Schneider1'2, Katharina Schulte3, and Patricio Novoa4 1 Botanik und Molekulare Evolutionsforschung, Senckenberg Gesellschaft für Naturforschung and Johann Wolfgang Goethe-Universität, Senckenberganlage 25, 60325, Frankfurt am Main, Germany; e-mail: [email protected]; e-mail: [email protected] 2 Biodiversity and Climate Research Center (BIK-F), Senckenberganlage 25, 60325, Frankfurt am Main, Germany 3 Australian Tropical Herbarium and Tropical Biodiversity and Climate Change Centre, James Cook University, PO Box 6811, Caims, QLD 4870, Australia; e-mail: [email protected] 4 Jardin Botânico Nacional, Camino El Olivar 305, El Salto, Vina del Mar, Chile Abstract. -

Water Relations of Bromeliaceae in Their Evolutionary Context
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Apollo Botanical Journal of the Linnean Society, 2016, 181, 415–440. With 2 figures Think tank: water relations of Bromeliaceae in their evolutionary context JAMIE MALES* Department of Plant Sciences, University of Cambridge, Downing Street, Cambridge CB2 3EA, UK Received 31 July 2015; revised 28 February 2016; accepted for publication 1 March 2016 Water relations represent a pivotal nexus in plant biology due to the multiplicity of functions affected by water status. Hydraulic properties of plant parts are therefore likely to be relevant to evolutionary trends in many taxa. Bromeliaceae encompass a wealth of morphological, physiological and ecological variations and the geographical and bioclimatic range of the family is also extensive. The diversification of bromeliad lineages is known to be correlated with the origins of a suite of key innovations, many of which relate directly or indirectly to water relations. However, little information is known regarding the role of change in morphoanatomical and hydraulic traits in the evolutionary origins of the classical ecophysiological functional types in Bromeliaceae or how this role relates to the diversification of specific lineages. In this paper, I present a synthesis of the current knowledge on bromeliad water relations and a qualitative model of the evolution of relevant traits in the context of the functional types. I use this model to introduce a manifesto for a new research programme on the integrative biology and evolution of bromeliad water-use strategies. The need for a wide-ranging survey of morphoanatomical and hydraulic traits across Bromeliaceae is stressed, as this would provide extensive insight into structure– function relationships of relevance to the evolutionary history of bromeliads and, more generally, to the evolutionary physiology of flowering plants.