Dermal Olfactory Receptor OR51B5 Is Essential for Survival and Collagen Synthesis in Human Dermal Fibroblast (Hs68 Cells)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Variation Across the Human Olfactory Receptor Repertoire Alters Odor Perception
bioRxiv preprint doi: https://doi.org/10.1101/212431; this version posted November 1, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Genetic variation across the human olfactory receptor repertoire alters odor perception Casey Trimmer1,*, Andreas Keller2, Nicolle R. Murphy1, Lindsey L. Snyder1, Jason R. Willer3, Maira Nagai4,5, Nicholas Katsanis3, Leslie B. Vosshall2,6,7, Hiroaki Matsunami4,8, and Joel D. Mainland1,9 1Monell Chemical Senses Center, Philadelphia, Pennsylvania, USA 2Laboratory of Neurogenetics and Behavior, The Rockefeller University, New York, New York, USA 3Center for Human Disease Modeling, Duke University Medical Center, Durham, North Carolina, USA 4Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, North Carolina, USA 5Department of Biochemistry, University of Sao Paulo, Sao Paulo, Brazil 6Howard Hughes Medical Institute, New York, New York, USA 7Kavli Neural Systems Institute, New York, New York, USA 8Department of Neurobiology and Duke Institute for Brain Sciences, Duke University Medical Center, Durham, North Carolina, USA 9Department of Neuroscience, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA *[email protected] ABSTRACT The human olfactory receptor repertoire is characterized by an abundance of genetic variation that affects receptor response, but the perceptual effects of this variation are unclear. To address this issue, we sequenced the OR repertoire in 332 individuals and examined the relationship between genetic variation and 276 olfactory phenotypes, including the perceived intensity and pleasantness of 68 odorants at two concentrations, detection thresholds of three odorants, and general olfactory acuity. -

An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors
Ecology and Evolutionary Biology 2021; 6(3): 53-77 http://www.sciencepublishinggroup.com/j/eeb doi: 10.11648/j.eeb.20210603.11 ISSN: 2575-3789 (Print); ISSN: 2575-3762 (Online) An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors Miguel Angel Fuertes*, Carlos Alonso Department of Microbiology, Centre for Molecular Biology “Severo Ochoa”, Spanish National Research Council and Autonomous University, Madrid, Spain Email address: *Corresponding author To cite this article: Miguel Angel Fuertes, Carlos Alonso. An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors. Ecology and Evolutionary Biology. Vol. 6, No. 3, 2021, pp. 53-77. doi: 10.11648/j.eeb.20210603.11 Received: April 24, 2021; Accepted: May 11, 2021; Published: July 13, 2021 Abstract: Capturing conserved patterns in genes and proteins is important for inferring phenotype prediction and evolutionary analysis. The study is focused on the conserved patterns of the G protein-coupled receptors, an important superfamily of receptors. Olfactory receptors represent more than 2% of our genome and constitute the largest family of G protein-coupled receptors, a key class of drug targets. As no crystallographic structures are available, mechanistic studies rely on the use of molecular dynamic modelling combined with site-directed mutagenesis data. In this paper, we hypothesized that human-mouse orthologs coding for G protein-coupled receptors maintain, at speciation events, shared compositional structures independent, to some extent, of their percent identity as reveals a method based in the categorization of nucleotide triplets by their gross composition. The data support the consistency of the hypothesis, showing in ortholog G protein-coupled receptors the presence of emergent shared compositional structures preserved at speciation events. -

A Framework to Identify Contributing Genes In
A framework to identify contributing genes in patients with Phelan-McDermid syndrome Anne-Claude Tabet, Thomas Rolland, Marie Ducloy, Jonathan Levy, Julien Buratti, Alexandre Mathieu, Damien Haye, Laurence Perrin, Céline Dupont, Sandrine Passemard, et al. To cite this version: Anne-Claude Tabet, Thomas Rolland, Marie Ducloy, Jonathan Levy, Julien Buratti, et al.. A frame- work to identify contributing genes in patients with Phelan-McDermid syndrome. npj Genomic Medicine, Springer Nature, 2019, 4 (1), pp.16. 10.1038/s41525-019-0090-y. hal-02347889 HAL Id: hal-02347889 https://hal.archives-ouvertes.fr/hal-02347889 Submitted on 16 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. bioRxiv preprint doi: https://doi.org/10.1101/117978; this version posted March 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. A framework to identify modifier genes in patients -

Misexpression of Cancer/Testis (Ct) Genes in Tumor Cells and the Potential Role of Dream Complex and the Retinoblastoma Protein Rb in Soma-To-Germline Transformation
Michigan Technological University Digital Commons @ Michigan Tech Dissertations, Master's Theses and Master's Reports 2019 MISEXPRESSION OF CANCER/TESTIS (CT) GENES IN TUMOR CELLS AND THE POTENTIAL ROLE OF DREAM COMPLEX AND THE RETINOBLASTOMA PROTEIN RB IN SOMA-TO-GERMLINE TRANSFORMATION SABHA M. ALHEWAT Michigan Technological University, [email protected] Copyright 2019 SABHA M. ALHEWAT Recommended Citation ALHEWAT, SABHA M., "MISEXPRESSION OF CANCER/TESTIS (CT) GENES IN TUMOR CELLS AND THE POTENTIAL ROLE OF DREAM COMPLEX AND THE RETINOBLASTOMA PROTEIN RB IN SOMA-TO- GERMLINE TRANSFORMATION", Open Access Master's Thesis, Michigan Technological University, 2019. https://doi.org/10.37099/mtu.dc.etdr/933 Follow this and additional works at: https://digitalcommons.mtu.edu/etdr Part of the Cancer Biology Commons, and the Cell Biology Commons MISEXPRESSION OF CANCER/TESTIS (CT) GENES IN TUMOR CELLS AND THE POTENTIAL ROLE OF DREAM COMPLEX AND THE RETINOBLASTOMA PROTEIN RB IN SOMA-TO-GERMLINE TRANSFORMATION By Sabha Salem Alhewati A THESIS Submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE In Biological Sciences MICHIGAN TECHNOLOGICAL UNIVERSITY 2019 © 2019 Sabha Alhewati This thesis has been approved in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE in Biological Sciences. Department of Biological Sciences Thesis Advisor: Paul Goetsch. Committee Member: Ebenezer Tumban. Committee Member: Zhiying Shan. Department Chair: Chandrashekhar Joshi. Table of Contents List of figures .......................................................................................................................v -

Research Article the Differentially Expressed
Ashdin Publishing Journal of Drug and Alcohol Research Vol. 10 (2021), Article ID 236125, 5 pages Research Article The Differentially Expressed Genes and Biomarker Identification for Dengue Disease Using Transcriptome Data Analysis Sunil Krishnan G, Amit Joshi and Vikas Kaushik* Department of Bioinformatics, Lovely Professional University, Punjab, India *Address Correspondence to: Vikas Kaushik, Department of Bioinformatics, Lovely Professional University, Punjab, India, E-mail: [email protected] Received: May 24, 2021; Accepted: June 07, 2021; Published: June 14, 2021 Copyright © 2021 Sunil Krishnan G. This is an open access article distributed under the terms of the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract [9]. Several microarray studies acknowledged differential- This bioinformatics and biostatistics study was designed to recognize and ly expressed genes (DEGs) from multiple sample profiles examine the differentially expressed genes (DEGs) linked with dengue vi- [10,11]. Consistently DEGs identified from various previ- rus infection in Homo sapiens. Thirty nine transcriptome profile datasets ous studies [12-16] were used to make out a potential bio- were analyzed by linear models for microarray analysis based on the R package of the biostatistics test for the identification of significantly ex- marker for the DENV disease. Meta-analysis approaches pressed genes associated with the disease. The Benjamini and Hochberg are common practice to discover novel DEG signatures for (BH) standard operating procedure assessed DEGs had the least false dis- superior biomarkers and synthetic/biotherapeutics [17,18]. covery rate and chosen for further bioinformatics gene analysis. -

Whole Genome Sequencing of Six Dog Breeds from Continuous Altitudes Reveals Adaption to High-Altitude Hypoxia
Downloaded from genome.cshlp.org on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press Whole genome sequencing of six dog breeds from continuous altitudes reveals adaption to high-altitude hypoxia Xiao Gou#1,2, Zhen Wang#3,4, Ning Li#2, Feng Qiu#4,5, Ze Xu 5, Dawei Yan 1, Shuli Yang 1 , Jia Jia 4, Xiaoyan Kong 1, Zehui Wei 6, Shaoxiong Lu 1, Linsheng Lian 1, Changxin Wu 2, Xueyan Wang 1, Guozhi Li 1, Teng Ma 1, Qiang Jiang 1, Xue Zhao 1, Jiaqiang Yang 1, Baohong Liu 5, Dongkai Wei 5, Hong Li 3,4, Jianfa Yang 1, Yulin Yan 1, Guiying Zhao 1, Xingxing Dong 1, Mingli Li 1, Weidong Deng 1, Jing Leng 1, Chaochun Wei 4,8, Chuan Wang 7, Huaming Mao*1, Hao Zhang*2, Guohui Ding*3,4, Yixue Li*3,4,8,9 1. College of Animal Science and Technology, Yunnan Agricultural University, Heilongtan, Northern district, Kunming 650201, China 2. College of Animal Science and Technology, China Agricultural University, 17 East Qinghua Road, Beijing 100083, China 3. Key Laboratory of Systems Biology, Shanghai Institutes for biological Sciences, Chinese Academy of Sciences, 320 Yueyang Road, Shanghai 200031, China 4. Shanghai Center for Bioinformation Technology, Shanghai Industrial Technology Institute, 1278 Keyuan Road, Shanghai 201203, China 5. EG Information Technology Enterprise (EGI), Encode Genomics Biotechnology Co., Ltd., 100 Qinzhou Road, Shanghai 200235, China 6. College of Animal Science and Technology, Northwest Agricultural and Forestry University, No.3 Taicheng Road, Yangling 712100, China 7. National Center for Protein Science • Shanghai, National Facility for Protein Science in Shanghai, Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, 320 Yueyang Road, Shanghai 200031, China 1 Downloaded from genome.cshlp.org on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press 8. -

Single Cell Derived Clonal Analysis of Human Glioblastoma Links
SUPPLEMENTARY INFORMATION: Single cell derived clonal analysis of human glioblastoma links functional and genomic heterogeneity ! Mona Meyer*, Jüri Reimand*, Xiaoyang Lan, Renee Head, Xueming Zhu, Michelle Kushida, Jane Bayani, Jessica C. Pressey, Anath Lionel, Ian D. Clarke, Michael Cusimano, Jeremy Squire, Stephen Scherer, Mark Bernstein, Melanie A. Woodin, Gary D. Bader**, and Peter B. Dirks**! ! * These authors contributed equally to this work.! ** Correspondence: [email protected] or [email protected]! ! Supplementary information - Meyer, Reimand et al. Supplementary methods" 4" Patient samples and fluorescence activated cell sorting (FACS)! 4! Differentiation! 4! Immunocytochemistry and EdU Imaging! 4! Proliferation! 5! Western blotting ! 5! Temozolomide treatment! 5! NCI drug library screen! 6! Orthotopic injections! 6! Immunohistochemistry on tumor sections! 6! Promoter methylation of MGMT! 6! Fluorescence in situ Hybridization (FISH)! 7! SNP6 microarray analysis and genome segmentation! 7! Calling copy number alterations! 8! Mapping altered genome segments to genes! 8! Recurrently altered genes with clonal variability! 9! Global analyses of copy number alterations! 9! Phylogenetic analysis of copy number alterations! 10! Microarray analysis! 10! Gene expression differences of TMZ resistant and sensitive clones of GBM-482! 10! Reverse transcription-PCR analyses! 11! Tumor subtype analysis of TMZ-sensitive and resistant clones! 11! Pathway analysis of gene expression in the TMZ-sensitive clone of GBM-482! 11! Supplementary figures and tables" 13" "2 Supplementary information - Meyer, Reimand et al. Table S1: Individual clones from all patient tumors are tumorigenic. ! 14! Fig. S1: clonal tumorigenicity.! 15! Fig. S2: clonal heterogeneity of EGFR and PTEN expression.! 20! Fig. S3: clonal heterogeneity of proliferation.! 21! Fig. -

The Potential Druggability of Chemosensory G Protein-Coupled Receptors
International Journal of Molecular Sciences Review Beyond the Flavour: The Potential Druggability of Chemosensory G Protein-Coupled Receptors Antonella Di Pizio * , Maik Behrens and Dietmar Krautwurst Leibniz-Institute for Food Systems Biology at the Technical University of Munich, Freising, 85354, Germany; [email protected] (M.B.); [email protected] (D.K.) * Correspondence: [email protected]; Tel.: +49-8161-71-2904; Fax: +49-8161-71-2970 Received: 13 February 2019; Accepted: 12 March 2019; Published: 20 March 2019 Abstract: G protein-coupled receptors (GPCRs) belong to the largest class of drug targets. Approximately half of the members of the human GPCR superfamily are chemosensory receptors, including odorant receptors (ORs), trace amine-associated receptors (TAARs), bitter taste receptors (TAS2Rs), sweet and umami taste receptors (TAS1Rs). Interestingly, these chemosensory GPCRs (csGPCRs) are expressed in several tissues of the body where they are supposed to play a role in biological functions other than chemosensation. Despite their abundance and physiological/pathological relevance, the druggability of csGPCRs has been suggested but not fully characterized. Here, we aim to explore the potential of targeting csGPCRs to treat diseases by reviewing the current knowledge of csGPCRs expressed throughout the body and by analysing the chemical space and the drug-likeness of flavour molecules. Keywords: smell; taste; flavour molecules; drugs; chemosensory receptors; ecnomotopic expression 1. Introduction Thirty-five percent of approved drugs act by modulating G protein-coupled receptors (GPCRs) [1,2]. GPCRs, also named 7-transmembrane (7TM) receptors, based on their canonical structure, are the largest family of membrane receptors in the human genome. -

OR51B4 (NM 033179) Human Untagged Clone Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for SC305589 OR51B4 (NM_033179) Human Untagged Clone Product data: Product Type: Expression Plasmids Product Name: OR51B4 (NM_033179) Human Untagged Clone Tag: Tag Free Symbol: OR51B4 Synonyms: HOR5'Beta1 Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin Fully Sequenced ORF: >NCBI ORF sequence for NM_033179, the custom clone sequence may differ by one or more nucleotides ATGTGGTATAACAACAGTGCTGGCCCCTTCTTGCTGACTGGCTTCTTGGGCTCAGAGGCAGTTCACTACC GGATCTCTATGTCCTTCTTTGTCATCTACTTCTCCGTCCTTTTTGGAAATGGCACTCTTCTTGTCCTCAT TTGGAATGATCACAGCCTCCATGAGCCCATGTACTACTTCCTGGCTATGCTGGCAGACACGGACCTTGGG ATGACATTCACTACAATGCCCACAGTCCTGGGTGTCCTGCTGCTAGACCAGAGGGAGATTGCCCATGCTG CCTGTTTCACCCAATCCTTCATTCATTCACTGGCCATTGTAGAATCAGGTATCTTGCTTGTTTTGGCCTA TGACTGTTTCATTGCCATCCGCACACCACTGAGGTACAACTGCATTCTTACCAATTCCCGAGTGATGAAC ATAGGACTGGGGGTACTGATGAGAGGTTTTATGTCCATTTTGCCCATAATTCTTTCACTCTACTGCTACC CATATTGTGGTTCCCGTGCCCTCTTGCACACATTTTGCCTCCATCAAGATGTCATAAAACTCGCCTGTGC TGATATCACGTTTAATCACATATATCCAATTATTCAGACTTCTTTGACTGTCTTTTTAGATGCTCTAATC ATCATCTTTTCTTATATACTAATCCTCAAGACAGTGATGGGCATTGCGTCTGGACAAGAGGAAGCTAAAT CTCTCAACACTTGTGTCTCCCATATTAGCTGTGTCCTAGTATTTCACATCACTGTGATGGGACTGTCATT CATTCACAGGTTTGGGAAACATGCACCTCATGTGGTCCCCATTACCATGAGCTATGTCCATTTTCTCTTT CCTCCATTCGTGAATCCTATCATTTATAGCATCAAGACCAAGCAGATTCAAAGAAGCATTATTCGCCTAT TTTCTGGGCAGAGTAGGGCTTGA -

The Mutational Landscape of Human Olfactory G Protein-Coupled Receptors
Jimenez et al. BMC Biology (2021) 19:21 https://doi.org/10.1186/s12915-021-00962-0 RESEARCH ARTICLE Open Access The mutational landscape of human olfactory G protein-coupled receptors Ramón Cierco Jimenez1,2, Nil Casajuana-Martin1, Adrián García-Recio1, Lidia Alcántara1, Leonardo Pardo1, Mercedes Campillo1 and Angel Gonzalez1* Abstract Background: Olfactory receptors (ORs) constitute a large family of sensory proteins that enable us to recognize a wide range of chemical volatiles in the environment. By contrast to the extensive information about human olfactory thresholds for thousands of odorants, studies of the genetic influence on olfaction are limited to a few examples. To annotate on a broad scale the impact of mutations at the structural level, here we analyzed a compendium of 119,069 natural variants in human ORs collected from the public domain. Results: OR mutations were categorized depending on their genomic and protein contexts, as well as their frequency of occurrence in several human populations. Functional interpretation of the natural changes was estimated from the increasing knowledge of the structure and function of the G protein-coupled receptor (GPCR) family, to which ORs belong. Our analysis reveals an extraordinary diversity of natural variations in the olfactory gene repertoire between individuals and populations, with a significant number of changes occurring at the structurally conserved regions. A particular attention is paid to mutations in positions linked to the conserved GPCR activation mechanism that could imply phenotypic variation in the olfactory perception. An interactive web application (hORMdb, Human Olfactory Receptor Mutation Database) was developed for the management and visualization of this mutational dataset. -

WO 2019/068007 Al Figure 2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/068007 Al 04 April 2019 (04.04.2019) W 1P O PCT (51) International Patent Classification: (72) Inventors; and C12N 15/10 (2006.01) C07K 16/28 (2006.01) (71) Applicants: GROSS, Gideon [EVIL]; IE-1-5 Address C12N 5/10 (2006.0 1) C12Q 1/6809 (20 18.0 1) M.P. Korazim, 1292200 Moshav Almagor (IL). GIBSON, C07K 14/705 (2006.01) A61P 35/00 (2006.01) Will [US/US]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., C07K 14/725 (2006.01) P.O. Box 4044, 7403635 Ness Ziona (TL). DAHARY, Dvir [EilL]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., P.O. (21) International Application Number: Box 4044, 7403635 Ness Ziona (IL). BEIMAN, Merav PCT/US2018/053583 [EilL]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., P.O. (22) International Filing Date: Box 4044, 7403635 Ness Ziona (E.). 28 September 2018 (28.09.2018) (74) Agent: MACDOUGALL, Christina, A. et al; Morgan, (25) Filing Language: English Lewis & Bockius LLP, One Market, Spear Tower, SanFran- cisco, CA 94105 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of national protection available): AE, AG, AL, AM, 62/564,454 28 September 2017 (28.09.2017) US AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, 62/649,429 28 March 2018 (28.03.2018) US CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, (71) Applicant: IMMP ACT-BIO LTD. -
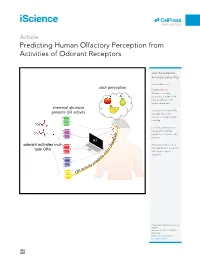
Predicting Human Olfactory Perception from Activities of Odorant Receptors
iScience ll OPEN ACCESS Article Predicting Human Olfactory Perception from Activities of Odorant Receptors Joel Kowalewski, Anandasankar Ray [email protected] odor perception HIGHLIGHTS Machine learning predicted activity of 34 human ORs for ~0.5 million chemicals chemical structure Activities of human ORs predicts OR activity could predict odor character using machine learning Few OR activities were needed to optimize r predictions of each odor e t c percept a AI r a odorant activates mul- h Behavior predictions in c Drosophila also need few r tiple ORs o olfactory receptor d o activities ts ic ed pr ity tiv ac OR Kowalewski & Ray, iScience 23, 101361 August 21, 2020 ª 2020 The Author(s). https://doi.org/10.1016/ j.isci.2020.101361 iScience ll OPEN ACCESS Article Predicting Human Olfactory Perception from Activities of Odorant Receptors Joel Kowalewski1 and Anandasankar Ray1,2,3,* SUMMARY Odor perception in humans is initiated by activation of odorant receptors (ORs) in the nose. However, the ORs linked to specific olfactory percepts are unknown, unlike in vision or taste where receptors are linked to perception of different colors and tastes. The large family of ORs (~400) and multiple receptors activated by an odorant present serious challenges. Here, we first use machine learning to screen ~0.5 million compounds for new ligands and identify enriched structural motifs for ligands of 34 human ORs. We next demonstrate that the activity of ORs successfully predicts many of the 146 different perceptual qualities of chem- icals. Although chemical features have been used to model odor percepts, we show that biologically relevant OR activity is often superior.