Characterization of Bronze Casting in Insula 56, in Roman Aventicum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Critical Review of Suitable Methods to Eliminate Mercury in Indonesia's Artisanal Gold Mining
[Type here] The University of British Columbia Norman B. Keevil Institute of Mining Engineering 6350 Stores Rd., Vancouver, BC, Canada, V6T 1Z4 ph: (604) 8224332, fax: (604) 8225599; [email protected] Report to UNDP Indonesia A Critical Review of Suitable Methods to Eliminate Mercury in Indonesia’s Artisanal Gold Mining: Co-existence is the Solution Marcello M. Veiga, PhD, PEng Professor For UNDP - United Nations Development Programme, Indonesia − March 2020 – A Critical Review of Suitable Methods to Eliminate Mercury in Indonesia’s AGM i Report to UNDP, March 2020, by Marcello M. Veiga _____________________________________________________________________________________ Table of Contents SUMMARY ...................................................................................................................................................... 1 1. MERCURY POLLUTION FROM ARTISANAL GOLD MINERS ..................................................................... 2 1.1. Some Definitions ............................................................................................................................ 2 1.2. Mercury in AGM in Indonesia ........................................................................................................ 3 1.3. Summary of the Main Problems of Mercury in AGM in Indonesia ............................................... 7 2. SOLUTIONS TRIED TO REDUCE/ELIMINATE MERCURY .......................................................................... 8 2.1. Environmental and Health Approach ........................................................................................... -

Occurrence and Extraction of Metals MODULE - 6 Chemistry of Elements
Occurrence and Extraction of Metals MODULE - 6 Chemistry of Elements 16 Notes OCCURRENCE AND EXTRACTION OF METALS Metals and their alloys are extensively used in our day-to-day life. They are used for making machines, railways, motor vehicles, bridges, buildings, agricultural tools, aircrafts, ships etc. Therefore, production of a variety of metals in large quantities is necessary for the economic growth of a country. Only a few metals such as gold, silver, mercury etc. occur in free state in nature. Most of the other metals, however, occur in the earth's crust in the combined form, i.e., as compounds with different anions such as oxides, sulphides, halides etc. In view of this, the study of recovery of metals from their ores is very important. In this lesson, you shall learn about some of the processes of extraction of metals from their ores, called metallurgical processes. OBJECTIVES After reading this lesson, you will be able to : z differentiate between minerals and ores; z recall the occurrence of metals in native form and in combined form as oxides, sulphides, carbonates and chlorides; z list the names and formulae of some common ores of Na, Al, Sn, Pb ,Ti, Fe, Cu, Ag and Zn; z list the occurrence of minerals of different metals in India; z list different steps involved in the extraction of metals; * An alloy is a material consisting of two or more metals, or a metal and a non-metal. For example, brass is an alloy of copper and zinc; steel is an alloy of iron and carbon. -

ENVIRONMENTAL CODE of PRACTICE Base Metals Smelters and Refineries
ENVIRONMENTAL CODE OF PRACTICE C ANADIAN E NVIRONMENTAL P ROTECTION A CT , 1999 First Edition Base Metals Smelters and Refineries March 2006 EPS 1/MM/11 E Metals Section Natural Resource Sectors Pollution Prevention Directorate Environmental Stewardship Branch Environment Canada Library and Archives Canada Cataloguing in Publication Main entry under title: Environmental Code of Practice for Base Metals Smelters and Refineries: Code of Practice, Canadian Environmental Protection Act, 1999. Issued also in French under title: Code de pratiques écologiques pour les fonderies et affineries de métaux communs : Code de pratique de la Loi canadienne sur la protection de l’environnement (1999). “First Edition”. Available also on the Internet. Includes bibliographical references. ISBN 0-662-42221-X Cat. no.: En84-34/2005E EPS 1/MM/11 E 1. Non-ferrous metal industries – Waste disposal – Canada. 2. Non-ferrous metals – Metallurgy – Environmental aspects – Canada. 3. Non-ferrous metals – Refining – Environmental aspects – Canada. 4. Smelting – Environmental aspects – Canada. 5. Best management practices (Pollution prevention) – Canada. i. Canada. Pollution Prevention Directorate. Metals Section. ii. Canada. Environment Canada. TD195.F6E58 2005 669'.028'6 C2005-980316-9 READERS’ COMMENTS Inquiries and comments on this Code of Practice, as well as requests for additional copies of the Code, should be directed to: Metals Section Natural Resources Sectors Division Pollution Prevention Directorate Environmental Stewardship Branch Environment Canada Place Vincent Massey 351 St. Joseph Blvd. Gatineau, Quebec K1A 0H3 Fax (819) 953-5053 Note: Website addresses mentioned in this document may have changed or references cited may have been removed from websites since the publication of the document. -
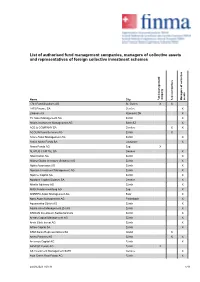
List of Authorised Fund Management Companies, Managers of Collective Assets and Representatives of Foreign Collective Investment Schemes
List of authorised fund management companies, managers of collective assets and representatives of foreign collective investment schemes Name City Fund management company Representatives Managers of collective assets 1741 Fund Solutions AG St. Gallen X X 1875 Finance SA Genève X 2Xideas AG Küsnacht ZH X 3V Asset Management AG Zürich X Abaris Investment Management AG Bäch SZ X ACE & COMPANY SA Genève X X ACOLIN Fund Services AG Zürich X Acoro Asset Management AG Zürich X Active Niche Funds SA Lausanne X Akara Funds AG Zug X ALATUS CAPITAL SA Genève X Albin Kistler AG Zürich X Allianz Global Investors (Schweiz) AG Zürich X Alpha Associates AG Zürich X Alpinum Investment Management AG Zürich X Alprime Capital AG Zürich X Alpstone Capital (Suisse) SA Genève X Altrafin Advisory AG Zürich X AMG Fondsverwaltung AG Zug X ANREPA Asset Management AG Baar X Apex Asset Management AG Freienbach X Aquamarine Zürich AG Zürich X Aquila Asset Management ZH AG Zürich X ARDIAN Investment Switzerland AG Zürich X Aretas Capital Management AG Zürich X Arete Ethik Invest AG Zürich X Arfina Capital SA Zürich X ARM Swiss Representatives SA Gland X Artico Partners AG Zürich X X Arvernus Capital AG Zürich X ARVEST Funds AG Zürich X AS Investment Management SARL Genève X Asia Green Real Estate AG Zürich X 24.09.2021 / 03:31 1/11 List of authorised fund management companies, managers of collective assets and representatives of foreign collective investment schemes Name City Fund management company Representatives Managers of collective assets Asteria Investment Managers SA Genève X Atlanticomnium SA Genève X AtonRâ Partners SA Genève X AUTONOMY CAPITAL RESEARCH TWO LIMITED, (St. -

The Roman Antiquities of Switzerland
Archaeological Journal ISSN: 0066-5983 (Print) 2373-2288 (Online) Journal homepage: http://www.tandfonline.com/loi/raij20 The Roman Antiquities of Switzerland By Bunnell Lewis M.A., F.S.A. To cite this article: By Bunnell Lewis M.A., F.S.A. (1885) The Roman Antiquities of Switzerland, Archaeological Journal, 42:1, 171-214, DOI: 10.1080/00665983.1885.10852174 To link to this article: http://dx.doi.org/10.1080/00665983.1885.10852174 Published online: 15 Jul 2014. Submit your article to this journal View related articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=raij20 Download by: [University of California Santa Barbara] Date: 18 June 2016, At: 04:49 THE ROMAN ANTIQUITIES OF SWITZERLAND. By BUNNELL LEWIS, M.A., F.S.A. Many persons, well-informed in other respects, think that there are no Boman antiquities in Switzerland. This mistake results from various causes. Most people travel there to enjoy the scenery, and recruit their health. The Bomans have not left behind them in that country vast monuments of their power, like the temples, theatres and aqueducts, which in regions farther south are still to be seen ; but, speaking generally, we must be content with smaller objects stored in museums, sometimes unprovided with catalogues.1 Moreover, no English writer, as far as I know, has discussed this subject at any length; attention has been directed almost exclusively to pie-historic remains made known by Dr. Keller's book on Bfahlbauten (lake- dwellings), of which an excellent translation has been published.2 However, I hope to show that the classical antiquities of Switzerland, though inferior to those of some other countries, ought not to be passed over with contemptuous neglect, and that they deserve study quite as much as similar relics of the olden time in Britain, 1 A very good account of the Collections 2 Dr. -

Refining of Non-Ferrous Metals
REFINING OF NON-FERROUS METALS J. G. BERRY- Abstract Fundamentals and Examples The fundamentals of refining of non - ferrous How, then, is the objective achieved ? It metals have been outlined . Examples of removal may be achieved by using the affinity of an electrolysis, of impurities by selective oxidation , undesirable element for another element, thus distillation , volatilization , etc., have been given. Refining of copper, lead, zinc, tin, and some other forming a compound, which is insoluble in the metals have been described . The importance of metal to be refined and which can easily be economics and time involved in refining process removed. Selective oxidation, electrolysis, has been stressed, distillation or volatilization may be used, while flotation, magnetic separation or chemi- cal reaction may be used to remove undesir- Introduction able elements prior to the reduction stage. T will be my endeavour in this paper to One of the earliest attempts at refining I indicate the fundamentals of refining would be the ` purification' of gold by as. they are applied to the more common thermal methods, while the most recent members of this group, with a description of development in this field is the solution and some of the processes, and, in doing so, I precipitation of metals under controlled hope to promote discussion which, after all, pressures from ores and concentrates or is the objective of this symposium. scrap, such as brass. In the fire-refining of copper; many im- purities are oxidized and removed in the Definition slag, these impurities being oxidized in pre- What, then, is the definition of ` refining ' ? ference to the copper itself. -

Silver City Santa Rita Hurley
SCENIC TRIPS to the GEOLOGIC PAST SILVER CITY SANTA RITA HURLEY NEW MEXICO Scenic Trips to the Geologic Past Series: No. I - Santa Fe, New Mexico No. 2 - Taos-Red River - Eagle Nest, New Mexico, Circle Drive No. 3 - Roswell- Capitan - Ruidoso and Bottomless Lakes Park, New Mexico No. 4 - Southern Zuni Mountains, New Mexico No. 5 - Silver City-Santa Rita-Hurley, New Mexico Additional copies of these guidebooks are available, for 25 cents, from the New Mexico Bureau of Mines and Mineral Resources, Campus Station, Socorro, New Mexico. HO: FOR THE GOLD AND SILVER MINES OF NEW MEXICO Fortune hunters, capitalists, poor men, Sickly folks, all whose hearts are bowed down; And Ye who would live long, be rich, healthy, and Happy; Come to our sunny clime and see For Yourselves. Handbill -- 1883 Santa Rita Open Pit, 1959. Scenic Trips to the Geologic Past No. 5 SILVER CITY - SANTA RITA - HURLEY, NEW MEXICO by JOHN H. SCHILLING 1959 STATE BUREAU OF MINES AND MINERAL RESOURCES a division of NEW MEXICO INSTITUTE OF MINING AND TECHNOLOGY Socorro - New Mexico NEW MEXICO INSTITUTE OF MINING AND TECHNOLOGY E. J. Workman, President STATE BUREAU OF MINES AND MINERAL RESOURCES Alvin J. Thompson, Director THE REGENTS Members Ex Officio The Honorable John Burroughs ...............Governor of New Mexico Tom Wiley ……….…… ...........Superintendent of Public Instruction Appointed Members Holm 0. Bursum, Jr. ........................................................ Socorro Thomas M. Cramer.......................................................... Carlsbad Frank C. DiLuzio ...................................................... Albuquerque John N. Mathews, Jr. ....................................................... Socorro Richard A. Matuszeski .............................................. Albuquerque PREFACE Much of the work undertaken at the New Mexico Bureau of Mines and Mineral Resources is done to help the mineral industries -- the prospector, miner, geologist, oil man. -

List of Swiss Cultural Goods: Archaeological Objects
List of Swiss cultural goods: archaeological objects A selection compiled by the Conference of Swiss Canton Archaeologists by order of the Federal Office of Culture FOC Konferenz schweizerischer Kantonsarchäologinnen und Kantonsarchäologen KSKA Conférence Suisse des Archéologues Cantonaux CSAC Conferenza Svizzera degli Archeologi Cantonali CSAC Categories of Swiss cultural goods I. Stone A. Architectural elements: made of granite, sandstone, marble and other types of stone. Capitals, window embrasures, mosaics etc. Approximate date: 50 BC – AD 1500. B. Inscriptions: on various types of stone. Altars, tombstones, honorary inscriptions etc. Approximate date: 50 BC – AD 800. C. Reliefs: on limestone and other types of stone. Stone reliefs, tomb- stone reliefs, decorative elements etc. Approximate date: mainly 50 BC – AD 800. D. Sculptures/statues: made of limestone, marble and other types of stone. Busts, statuettes, burial ornaments etc. Approximate date: mainly 50 BC – AD 800. E. Tools/implements: made of flint and other types of stone. Various tools such as knife and dagger blades, axes and implements for crafts etc. Approximate date: 130 000 BC – AD 800. F. Weapons: made of shale, flint, limestone, sandstone and other types of stone. Arrowheads, armguards, cannonballs etc. Approxi- mate date: 10 000 BC – AD 1500. G. Jewellery/accessories: made of various types of stone. Pendants, beads, finger ring inlays etc. Approximate date: mainly 2800 BC – AD 800. II. Metal A. Statues /statuettes / made of non-ferrous metal, rarely from precious metal. busts: Depictions of animals, humans and deities, portrait busts etc. Approximate date: 50 BC – AD 800. B. Vessels: made of non-ferrous metal, rarely from precious metal and iron. -

Principles of Metallurgy
Chapter 13 Principles of Metallurgy In class 8 you have studied about certain properties of metals like, malleability, ductility, sonarity etc. Metals play an important role in our daily life. We use various metals for various purposes like gold and silver as jewellary, copper, iron, alluminium for making conducting wires and for making utensils etc. We use many house hold articles made up of metals and their alloys at our home. • Can you mention some articles that are made up of metals? • Do metals exist in nature in the form same as that we use in our daily life? • Have you ever heard the words like ore, mineral and metallurgy? • Do you know how these metals are obtained? To understand these questions you need to know about metallurgy. In this chapter we discuss various concepts related to metallurgy and the process by which we are able to obtain the pure form of metal that we use in our daily life. “Metallurgy is the process of extraction of metals from their ores”. Human history in terms of materials had the Bronze Age and Iron Age pertaining to the metals they started to use the bronze (an alloy of copper and tin) and iron. Now we have more than 75% metals among the elements available. Occurrence of the metals in nature • How the metals are present in nature? The earth’s crust is the major source of metals. Sea water also contains some soluble salts such as sodium chloride and magnesium chloride etc. 286 X Class Principles of Metallurgy Some metals like gold (Au), silver (Ag) and copper (Cu) are available in nature in free state (native) as they are least reactive. -

WC/97/014 a Review of Gold Particle-Size and Recovery Methods
British Geological Survey TECHNICAL REPORT WC/97/14 Overseas Geology Series A REVIEW OF GOLD-PARTICLE-SIZE AND RECOVERY METHODS CJ Mitchell, EJ Evans & M T Styles This document is an output of a project funded by the UK Overseas Development Administration (ODA) for the benefit of developing countries. The views expressed in this document are not necessarily those of the ODA. ODA Classi$cation Subsector: Geoscience Theme: G2, Identify and ameliorate minerals-related and other geochemical toxic hazards Project Title: Mitigation of mining-related mercury pollution hazards Reference number: R6226 Bibliographic reference: C J Mitchell, E J Evans & M T Styles, A review of gold particle-size and recovery methods BGS Technical Report WC/97/14 Keywords: Gold, mineral processing, Malaysia, Zimbabwe, New Zealand, Papua New Guinea, Canada Front cover illustration: Processing of gold ore using a sluice box, Malaysia 0NERC 1997 Keyworth, Nottingham, British Geological Survey, 1997 Mitigation of mining-related mercury pollution hazards BRITISH GEOLOGICAL SURVEY Mineralogy & Petrology Group Technical Report WC/97/14 A REVIEW OF GOLD PARTICLE-SIZE AND RECOVERY METHODS CJ Mitchell, EJ Evans & MT Styles 1. INTRODUCTION This report reviews published literature concerning the particle-size distribution of gold occurring in alluvial and bed-rock deposits and the various methods used for its recovery. The aim of this review is to recommend methods for the recovery of fine- grained gold (generally less than 100 pm in size) as an alternative to the environmentally damaging use of mercury amalgamation. This work has been carried out as part of an ODA / BGS Technology Development Research (TDR) project R6226 "Mitigation of mining-related mercury pollution hazards". -

General Principles and Processes of Isolation of Elements
General principles and processes of isolation of elements CHAPTER GENERAL PRINCIPLES AND PROCESSES 7 OF ISOLATION OF ELEMENTS LEARNING OBJECTIVES (i) Explain the terms minerals, ores, concentration, benefaction, calcination, roasting, refining, etc. (ii) Understand the principles of oxidation and reduction as applied to the extraction procedures. (iii) Apply the thermodynamic concepts like that of Gibbs energy and entropy to the principles of extraction of Al, Cu, Zn and Fe. (iv) Explain why reduction of certain oxides like Cu2O is much easier than that of Fe2O3. (iv) Explain why CO is a favourable reducing agent at certain temperatures while coke is better in some other cases. (v) Explain why specific reducing agents are used for the reduction purposes. INTRODUCTION : Metals occur in nature, sometimes in free or native state but mostly in combined state i.e., in the form of their stable compounds associated with gangue or matrix. The earthy impurities such as sand, clay, rocks etc. associated with ore are collectively known as gangue or matrix. Metals like Cu, Ag, Au and Pt occur in the native form (metallic state). The natural material in which the metallic compounds (in the combined state) occur in the earth’s crust is known as mineral. A mineral may be a single compound or a complex mixture. Those minerals from which the metal can be readily and economically extracted are called ores. Thus all ores are minerals but all minerals are not ores. For eg. , bauxite (Al2O3 . 2H2O) and clay (Al2O3 . 2 SiO2 . 2H2O) are two minerals of aluminium, but aluminium can be profitably extracted only from bauxite and not from clay. -

Occurrence and Extraction of Metals MODULE - 6 Chemistry of Elements
Occurrence and Extraction of Metals MODULE - 6 Chemistry of Elements 18 Notes OCCURRENCE AND EXTRACTION OF METALS Metals and their alloys are extensively used in our day-to-day life. They are used for making machines, railways, motor vehicles, bridges, buildings, agricultural tools, aircrafts, ships etc. Therefore, production of a variety of metals in large quantities is necessary for the economic growth of a country. Only a few metals such as gold, silver, mercury etc. occur in free state in nature. Most of the other metals, however, occur in the earth's crust in the combined form, i.e., as compounds with different anions such as oxides, sulphides, halides etc. In view of this, the study of recovery of metals from their ores is very important. In this lesson, you shall learn about some of the processes of extraction of metals from their ores, called metallurgical processes. Objectives After reading this lesson, you will be able to : differentiate between minerals and ores; recall the occurrence of metals in native form and in combined form as oxides, sulphides, carbonates and chlorides; list the names and formulae of some common ores of Na, Al, Sn, Pb ,Ti, Fe, Cu, Ag and Zn; list the occurrence of minerals of different metals in India; list different steps involved in the extraction of metals; * An alloy is a material consisting of two or more metals, or a metal and a non-metal. For example, brass is an alloy of copper and zinc; steel is an alloy of iron and carbon. 1 MODULE - 6 Chemistry Chemistry of Elements list and explain various methods for concentration of ores (gravity separation, magnetic separation, froth floatation and chemical method); explain different metallurgical operations : roasting, calcination and smelting with suitable examples; choose the reducing agent for a given ore; Notes differentiate between flux and slag, and explain different methods for refining of metals : poling, liquation, distillation and electrolytic refining.