2017 Forbes-Harper Et Al Fox Skull Ecomorphology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
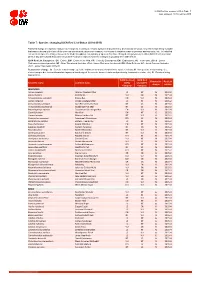
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Dental and Temporomandibular Joint Pathology of the Kit Fox (Vulpes Macrotis)
Author's Personal Copy J. Comp. Path. 2019, Vol. 167, 60e72 Available online at www.sciencedirect.com ScienceDirect www.elsevier.com/locate/jcpa DISEASE IN WILDLIFE OR EXOTIC SPECIES Dental and Temporomandibular Joint Pathology of the Kit Fox (Vulpes macrotis) N. Yanagisawa*, R. E. Wilson*, P. H. Kass† and F. J. M. Verstraete* *Department of Surgical and Radiological Sciences and † Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis, California, USA Summary Skull specimens from 836 kit foxes (Vulpes macrotis) were examined macroscopically according to predefined criteria; 559 specimens were included in this study. The study group consisted of 248 (44.4%) females, 267 (47.8%) males and 44 (7.9%) specimens of unknown sex; 128 (22.9%) skulls were from young adults and 431 (77.1%) were from adults. Of the 23,478 possible teeth, 21,883 teeth (93.2%) were present for examina- tion, 45 (1.9%) were absent congenitally, 405 (1.7%) were acquired losses and 1,145 (4.9%) were missing ar- tefactually. No persistent deciduous teeth were observed. Eight (0.04%) supernumerary teeth were found in seven (1.3%) specimens and 13 (0.06%) teeth from 12 (2.1%) specimens were malformed. Root number vari- ation was present in 20.3% (403/1,984) of the present maxillary and mandibular first premolar teeth. Eleven (2.0%) foxes had lesions consistent with enamel hypoplasia and 77 (13.8%) had fenestrations in the maxillary alveolar bone. Periodontitis and attrition/abrasion affected the majority of foxes (71.6% and 90.5%, respec- tively). -

An Educator's Resource to Texas Mammal Skulls and Skins
E4H-014 11/17 An Educator’s Resource to Texas Mammal Skulls and Skins for use in 4-H Wildlife Programs and FFA Wildlife Career Development Events By, Denise Harmel-Garza Program Coordinator I, Texas A&M AgriLife Extension Service, 4-H Photographer and coauthor, Audrey Sepulveda M.Ed. Agricultural Leadership, Education and Communications, Texas A&M University College Station, Texas 2017 “A special thanks to the Biodiversity Research and Teaching Collections at Texas A&M University for providing access to their specimens.” Texas A&M AgriLife Extension provides equal opportunities in its programs and employment to all persons, regardless of race, color, sex, religion, national origin, disability, age, genetic information, veteran status, sexual orientation, or gender identity. The Texas A&M University System, U.S. Department of Agriculture, and the County Commissioners Courts of Texas Cooperating. Introduction Texas youth that participate in wildlife programs may be asked to identify a skull, skin, scat, tracks, etc. of an animal. Usually, educators must find this information and assemble pictures of skulls and skins from various sources. They also must ensure that what they find is relevant and accurate. Buying skulls and skins to represent all Texas mammals is costly. Most educators cannot afford them, and if they can, maintaining these collections over time is problematic. This study resource will reduce the time teachers across the state need to spend searching for information and allow them more time for presenting the material to their students. This identification guide gives teachers and students easy access to information that is accurate and valuable for learning to identify Texas mammals. -

H420/02 Biological Diversity
A Level Biology A H420/02 Biological Diversity Question Set 11 1 The cheetah, Acinonyx jubatus, is a member of the cat family, Felidae. Cheetahs display less intraspecific variation than other members of the family Felidae. Fig. 20.1 shows the mean body length of a population of cheetahs from southern Africa. The error bars on Fig. 20.1 show the standard deviation of mean body length. Fig. 20.1 (a) (i) At between 2.5 and 4 years old, the mean length of female cheetahs is less than that of males. Calculate how much shorter than males female cheetahs are. Show your working. Express your answer as a percentage to two significant figures. Answer……………% [2] (ii) Using only Fig. 20.1 and your answer to (i), what can be concluded about the significance of the difference between the length of male and female cheetahs aged between 2.5 and 4 years? Explain your answer. [2] (iii) A student looked at Fig. 20.1 and wrote: “The longest male cheetah that was measured was 1.52 m long”. Explain whether the information in Fig. 20.1 supports the student’s answer. [1] (iv) State the likely causes of variation in body length in cheetahs. [2] (b) The population of cheetahs has been declining for the past 100 years and is estimated to be between 6000 and 7000. Within the remaining cheetah population, intraspecific genetic diversity is very low. One isolated population of cheetahs in Iran has fewer than 100 individuals. (i) State one way in which genetic diversity can be measured. -

Comparative Craniometric Measurements of Two Sympatric Species of Vulpes in Ikh Nart Nature Reserve, Mongolia
© 2018 Journal compilation ISSN 1684-3908 (print edition) http://mjbs.num.edu.mn Mongolian Journal of Biological http://biotaxa.org./mjbs Sciences MJBS Volume 16(1), 2018 ISSN 2225-4994 (online edition) http://dx.doi.org/10.22353/mjbs.2018.16.03 Original Article Comparative Craniometric Measurements of Two Sympatric Species of Vulpes in Ikh Nart Nature Reserve, Mongolia Tserendorj Munkhzul1, Richard P. Reading2, Bayarbaatar Buuveibaatar3 & James D. Murdoch4 1Mammalian Ecology Laboratory, Institute of General and Experimental Biology, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia 2International Conservation Coalition, Denver, Colorado 80220 USA, Butterfly Pavilion, Westminster, Colorado 80020 USA & Mongolian Conservation Coalition, Ulaanbaatar, Mongolia 3Wildlife Conservation Society, Mongolia Program, Ulaanbaatar, Mongolia 4Rubenstein School of Environment and Natural Resources, University of Vermont, George Aiken Center, Burlington, Vermont 05405 USA Abstract Key words: Corsac fox; In Mongolia, both the red fox (Vulpes vulpes) and corsac fox (Vulpes corsac) occupy cranium; morphometry; broad sympatric ranges, but despite their expansive ranges, few published details of red fox; Vulpes; skull. the craniometry of either species exist in Mongolia and other parts of northern and Article information: central Asia. To determine the morphological differences between two species of Received: 08 Febr. 2018 foxes, we tested for morphological and morphometrical differences between the red Accepted: 31 May 2018 (n = 13) and corsac (n = 11) foxes using 63 cranium measurements. All significantly Published online: different skull variables were larger for red foxes than corsac foxes. This paper 12 June 2018 reports comparison of the cranial measurements from skulls of red and corsac foxes Correspondence: and serves as a preliminary investigation of interspecific variation between these [email protected] species. -

Return of a Lost Structure in the Evolution of Felid Dentition Revisited: a Devoevo Perspective on the Irreversibility of Evolution
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429820; this version posted February 5, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. RUNNING HEAD: LYNX M2 AND IRREVERSIBILE EVOLUTION Return of a lost structure in the evolution of felid dentition revisited: A DevoEvo perspective on the irreversibility of evolution Vincent J. Lynch Department of Biological Sciences, University at Buffalo, SUNY, 551 Cooke Hall, Buffalo, NY, 14260, USA. Correspondence: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429820; this version posted February 5, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. RUNNING HEAD: LYNX M2 AND IRREVERSIBILE EVOLUTION Abstract There is a longstanding interest in whether the loss of complex characters is reversible (so-called “Dollo’s law”). Reevolution has been suggested for numerous traits but among the first was Kurtén (1963), who proposed that the presence of the second lower molar (M2) of the Eurasian lynx (Lynx lynx) was a violation of Dollo’s law because all other Felids lack M2. While an early and often cited example for the reevolution of a complex trait, Kurtén (1963) and Werdelin (1987) used an ad hoc parsimony argument to support their proposition that M2 reevolved in Eurasian lynx. Here I revisit the evidence that M2 reevolved in Eurasian lynx using explicit parsimony and maximum likelihood models of character evolution and find strong evidence that Kurtén (1963) and Werdelin (1987) were correct – M2 reevolved in Eurasian lynx. -

Anatomy of the Red Panda (Ailurus Fulgens)
Journal of Biology and Life Science ISSN 2157-6076 2019, Vol. 10, No. 1 Anatomy of the Red Panda (Ailurus fulgens) Modesta Makungu (Corresponding Author) Department of Veterinary Surgery and Theriogenology, College of Veterinary Medicine and Biomedical Sciences, Sokoine University of Agriculture, P O Box 3020, Morogoro, Tanzania. Email: [email protected]; [email protected] Received: September 21, 2018 Accepted: October 19, 2018 doi:10.5296/jbls.v10i1.13677 URL: https://doi.org/10.5296/jbls.v10i1.13677 Abstract The red panda (Ailurus fulgens) is an endangered species primarily distributed in the southern China and Himalayas. It lives in mountain forests with bamboo understory. This review outlines the normal anatomy of the red panda in terms of its musculoskeletal system, respiratory system, circulatory system, digestive system and urogenital system. Knowledge of the normal anatomy of individual animal species is important for species identification and accurate interpretation and diagnosis of diseases. Keywords: anatomy, red panda 1. Introduction The red panda (Ailurus fulgens) (Figure 1) is classified as an endangered species by the International Union for Conservation of Nature and Natural Resources (IUCN, 2018). It belongs to order; Carnivora, family; Ailuridae, and genus; Ailurus (Wei and Zhang, 2009; IUCN, 2018). The red panda is the only living species of the family Ailuridae and genus Ailurus and is closely related to mustelids, procyonids and skunks (Wei and Zhang, 2009; Groves, 2011). 13 Journal of Biology and Life Science ISSN 2157-6076 2019, Vol. 10, No. 1 Figure 1. A photograph of an adult red panda (Ailurus fulgens fulgens) It is primarily distributed in India, Burma, China, Bhutan and Nepal (Roberts and Gittleman, 1984; Ghose and Dutta, 2011). -

Scapular Form in Semi-Arboreal and Terrestrial Carnivores: How Climbing Affects the Shape of the Scapula
Scapular Form in Semi-Arboreal and Terrestrial Carnivores: How Climbing Affects the Shape of the Scapula Ashley Wells The Scapula http://www.exerciseology.me/doug_kelseys_blog/2009/01/d etails-on-thoracic-spine-flexibility.html Introduction Procyon lotor Urocyon cinereoargenteus Vulpes vulpes Introduction Procyon lotor Urocyon cinereoargenteus Vulpes vulpes Introduction Procyon lotor Urocyon cinereoargenteus Vulpes vulpes Introduction Procyon lotor Urocyon cinereoargenteus Vulpes vulpes Order Carnivora Suborder Caniformia http://susasverige.se/Calle/metazoa/laurasiatheria.htm How is this Anthropology? Biological anthropology ◦ Skeletal morphology ◦ Methods Zooarchaeology ◦ Apply to fossil remains http://www.mesacc.edu/dept/d10/ asb/origins/primates/index.html Research Questions Can the same patterns found in primate scapular morphology associated with locomotion be found in these carnivores? A large supraspinous fossa area is found in arboreal species A large infraspinous fossa area is found in terrestrial quadrupeds Methods Collection was from the Illinois State Museum Three-dimensional coordinate data were recorded for 10 landmarks The landmarks were then converted into 18 lengths ◦ size adjusted Differences in lengths were tested for using analysis of variance http://www.emicroscribe.com/products/solutions-for-anthropology-and- paleontology/typical-set-up-for-physical-anthropologist.htm Methods Collection was from the Illinois State Museum Three-dimensional coordinate data were recorded for 10 landmarks The landmarks were then converted into 18 lengths ◦ size adjusted Differences in lengths were tested for using analysis of variance Results Red vs gray Between red and gray fox ◦ Gray fox is significantly larger on the medial supraspinous border (B-C). ◦ Red fox is significantly larger for both the infraspinous vertebral border (A-B) and the lateral supraspinous border (C-D). -

Brain, Craniofacial, and Dental Lesions of a Free-Ranging Gray Wolf (Canis Lupus) Implicated in a Human Attack in Minnesota, USA
DOI: 10.7589/2015-01-014 Journal of Wildlife Diseases, 52(1), 2016, pp. 131–137 # Wildlife Disease Association 2016 Brain, Craniofacial, and Dental Lesions of a Free-ranging Gray Wolf (Canis lupus) Implicated in a Human Attack in Minnesota, USA Marc Schwabenlander,1 Kevin Stepaniuk,2 Michelle Carstensen,3 and Aníbal G. Armién1,4,51Veterinary Diagnostic Laboratory, College of Veterinary Medicine, University of Minnesota, 1333 Gortner Avenue, St. Paul, Minnesota 55108, USA; 2Veterinary Clinical Sciences, College of Veterinary Medicine, University of Minnesota, 1365 Gortner Avenue, St. Paul, Minnesota 55108, USA; 3Minnesota Department of Natural Resources, 5463C W Broadway, Forest Lake, Minnesota 55025, USA; 4Veterinary Population Medicine, College of Veterinary Medicine, University of Minnesota, 1365 Gortner Avenue, St. Paul, Minnesota 55108, USA; 5Corresponding author (email: [email protected]) ABSTRACT: We describe significant brain, University of Minnesota Veterinary Diag- craniofacial, and dental lesions in a free- nostic Laboratory (St. Paul, Minnesota, ranging wolf (Canis lupus) involved in USA) for identity confirmation and post- a human attack. On postmortem examination, the wolf presented asymmetric atrophy and mortem examination. bone remodeling affecting the mandible, The wolf was a 37-kg, approximately 1.5- incisive, maxilla, lacrimal, palatine, frontal, and yr-old intact male. On postmortem exami- ethmoid bones. There was an asymmetrical nation, the animal showed facial deformity. skeletal malocclusion and dental abnormali‐ ties including rotated, malpositioned, partially It was severely emaciated with a mild erupted teeth, and an odontogenic cyst asso‐ amount of subcutaneous and inner abdom- ciated with an unerupted canine tooth. Brain inal adipose tissue. The esophagus was changes were bilateral loss and atrophy of empty, the stomach contained fish bones extensive cortex regions including olfactory and scales, and the intestines had semifluid bulb, peduncles, and tract, and the frontal lobe. -

When Carnivores Roam: Temporal Patterns and Overlap Among Madagascar’S Native and Exotic Carnivores Z
bs_bs_bannerJournal of Zoology Journal of Zoology. Print ISSN 0952-8369 When carnivores roam: temporal patterns and overlap among Madagascar’s native and exotic carnivores Z. J. Farris1, B. D. Gerber2, S. Karpanty1, A. Murphy1, V. Andrianjakarivelo3, F. Ratelolahy3 & M. J. Kelly1 1 Department of Fish and Wildlife Conservation, Virginia Tech, Blacksburg, VA, USA 2 Colorado Cooperative Fish and Wildlife Research Unit, Department of Fish, Wildlife, and Conservation Biology, Colorado State University, Fort Collins, CO, USA 3 Wildlife Conservation Society Madagascar Program, Antananarivo, Madagascar Keywords Abstract activity; conservation; domestic dog; feral cat; fossa; invasive species; niche; season. Madagascar’s Eupleridae carnivores are perhaps the least studied and most threatened family of Carnivora. Investigating potential direct and indirect com- Correspondence petition among these native species and sympatric exotic carnivores is necessary to Zach J. Farris, Department of Fish and better direct conservation actions. From 2008 to 2013, we photographically sur- Wildlife Conservation, Virginia Tech, 124 veyed a diverse rainforest landscape, comparing six native and three exotic carni- Cheatham Hall, Blacksburg, VA 24060, USA. vores’ activity patterns throughout the diel cycle. We used hierarchical Bayesian Email: [email protected] Poisson analysis to describe the activity patterns of Madagascar’s carnivore com- munity, assessed effects of season and site on temporal activity patterns, and Editor: Nigel Bennett estimated coefficients of overlap between carnivore pairings to assess effects of body size and ecological niche on temporal overlap among native and exotic Received 29 September 2014; revised 20 carnivores. We observed changes in temporal activity patterns across seasons November 2014; accepted 15 December particularly during the austral summer (hot–dry season) for four native and two 2014 exotic carnivores, including evidence of fossa Cryptoprocta ferox altering their temporal activity during their mating season (hot–dry season). -

FOSSA CARNIVORA Family: Eupleridae Genus: Cryptoprocta Species: Ferox
FOSSA CARNIVORA Family: Eupleridae Genus: Cryptoprocta Species: ferox Range: endemic to Madagascar Habitat: prefers pristine undisturbed forests; it prefers humid over dry forests Niche: cathemeral, terrestrial & arboreal, carnivorous Wild diet: over 50% of diet is lemurs, also tenrecs, rodents, lizards and birds Zoo diet: Life Span: (Wild) 15 years (Captivity) 20+ yrs Sexual dimorphism: males larger than females Location in SF Zoo: APPEARANCE & PHYSICAL ADAPTATIONS: The fossa is the largest carnivorous mammal of Madagascar; it is endemic to the island of Madagascar. They have slender bodies, muscular limbs, and short, reddish-brown coats. They have small, cat-like heads, short, dog-like muzzles, and large, rounded ears. They are agile climbers with semi-retractable claws and flexible ankles that allow them to climb up and down trees head-first, and also support jumping from tree to tree. The fossa has a plantigrade gait among the trees, which gives it extra balance and stability when leaping. The fossa's tail makes up about half of the animal's length and provides balance while hunting and maneuvering amongst the trees. Weight: M 14 – 19 lbs F 12 – 15 lbs The male fossa has external genitalia with an unusually long penis HRL: 23 – 30 in and baculum (penis bone) with a spiny shaft. Unique to fossa, the female TL: 22 – 28 in undergoes a strange developmental stage during adolescence (about 1-2 years) known as transient masculinization. She develops an enlarged, spiky clitoris that resembles the male’s penis, and secretes an orange substance on her underbelly, which is otherwise seen only in mature males. -

Endangered Species Day Celebrate Endangered Species Day at Omaha’S Henry Doorly Zoo and Aquarium®
Endangered Species Day Celebrate Endangered Species Day at Omaha’s Henry Doorly Zoo and Aquarium®. Take yourself on a self-guided tour through the Zoo to learn about these amazing animals! African Grasslands Expedition Madagascar Lied Jungle® Suzanne and Walter Scott Okapi Madagascar teal Northern white cheeked Aquarium Giraffe Radiated tortoise gibbon Loggerhead turtle Cheetah Fossa Greater slow loris Southern giant clam Plains zebra Aye aye Cotton headed tamarin Southern rockhopper Bongo African straw colored fruit bat Bairds tapir penguin African lion Collared brown lemur Malayan tapir Atlantic puffin African elephant Crowned lemur Fancois langur Giant grouper Mongoose lemur Painted terrapin Red grouper Butterfly and Insect Malagasy giant jumping rat Soa soa water lizard Coral cat shark Pavilion Ring tailed lemur Home hinge back turtle Cactus coral Madagascar crested ibis Malaysian giant turtle Red knee tarantula Black grouper Parkers golden frog Yellow spotted river turtle Salt creek tiger beetle Zebra shark Green golden frog Pig nosed turtle White cockatoo Atlantic green sea turtle Standings day gecko African softshell turtle Crested partridge Goliath grouper Marbles rain frog Panamanian golden frog Horseshoe crab Cat Complex Golden matella Giant thai catfish Black botched stingray Amur tiger Clown knife fish Staghorn coral Black handed spider monkey Garden of the Senses Aruli barb Red panda (animals on display are weather Golden dragon fish dependent) Komodo dragon Yellow headed amazon Animals Off Display: Mutual of Omaha’s Wild