Seasonal Changes in Mollusc Abundance in a Tropical Intertidal Ecosystem, Banc D'arguin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moluscos Del Perú
Rev. Biol. Trop. 51 (Suppl. 3): 225-284, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Moluscos del Perú Rina Ramírez1, Carlos Paredes1, 2 y José Arenas3 1 Museo de Historia Natural, Universidad Nacional Mayor de San Marcos. Avenida Arenales 1256, Jesús María. Apartado 14-0434, Lima-14, Perú. 2 Laboratorio de Invertebrados Acuáticos, Facultad de Ciencias Biológicas, Universidad Nacional Mayor de San Marcos, Apartado 11-0058, Lima-11, Perú. 3 Laboratorio de Parasitología, Facultad de Ciencias Biológicas, Universidad Ricardo Palma. Av. Benavides 5400, Surco. P.O. Box 18-131. Lima, Perú. Abstract: Peru is an ecologically diverse country, with 84 life zones in the Holdridge system and 18 ecological regions (including two marine). 1910 molluscan species have been recorded. The highest number corresponds to the sea: 570 gastropods, 370 bivalves, 36 cephalopods, 34 polyplacoforans, 3 monoplacophorans, 3 scaphopods and 2 aplacophorans (total 1018 species). The most diverse families are Veneridae (57spp.), Muricidae (47spp.), Collumbellidae (40 spp.) and Tellinidae (37 spp.). Biogeographically, 56 % of marine species are Panamic, 11 % Peruvian and the rest occurs in both provinces; 73 marine species are endemic to Peru. Land molluscs include 763 species, 2.54 % of the global estimate and 38 % of the South American esti- mate. The most biodiverse families are Bulimulidae with 424 spp., Clausiliidae with 75 spp. and Systrophiidae with 55 spp. In contrast, only 129 freshwater species have been reported, 35 endemics (mainly hydrobiids with 14 spp. The paper includes an overview of biogeography, ecology, use, history of research efforts and conser- vation; as well as indication of areas and species that are in greater need of study. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

An Experimental Analysis of the Escape Response of the Gastropod
Pacific Science(1977), Vol. 31, No.1, p. 1-11 Printed in Great Britain An Experimental Analysis of the Escape Response of the Gastropod Strombus maculatus I LAURENCE H. FIELD 2 ABSTRACT: The escape response of Strombus maculatus is described in detail, including the apparent adaptive morphology of the foot, operculum, and eyestalks. The response is elicited by a chemical stimulus from two molluscivorous species of Conus and two gastropod-eating species of Cymatium but not from other pre datory species of these genera. Strombus habituated within three trials to a solution of "factor" from Conus pennaceus, but habituated only rarely, and then only after many trials, to contact with the live Conus. It was concluded that the eyes of S. maculatus are not used to see the Conus; however, eye removal significantly disrupted the orientation of the escape response, suggesting that the animal monitors some environmental cue such as polarized light. Tentacle removal appeared to interfere with escape response orientation but only to a variable extent. HERBIVOROUS GASTROPODS exhibit distinctive posterior end of the foot is thrust against the escape behavior from sea stars (Bauer 1913, substrate, causing the shell and head to lunge Feder and Christensen 1966) and predatory forward. Little additional work has been done gastropods (reviews by Kohn 1961, Robertson on strombid locomotion, and as Kohn and 1961, Kahn and Waters 1966, Gonor 1965, Waters (1966: 341) indicated, "the component 1966). 'Strombus has the remarkable ability to steps of the process have not been analyzed and escape from predators by rapid lunges, using the the functional morphology remains to be operculum to push against the substrate (Kohn studied in detail." Recently Berg (1972) and Waters 1966). -

FRESHWATER CRABS in AFRICA MICHAEL DOBSON Dr M
CORE FRESHWATER CRABS IN AFRICA 3 4 MICHAEL DOBSON FRESHWATER CRABS IN AFRICA In East Africa, each highland area supports endemic or restricted species (six in the Usambara Mountains of Tanzania and at least two in each of the brought to you by MICHAEL DOBSON other mountain ranges in the region), with relatively few more widespread species in the lowlands. Recent detailed genetic analysis in southern Africa Dr M. Dobson, Department of Environmental & Geographical Sciences, has shown a similar pattern, with a high diversity of geographically Manchester Metropolitan University, Chester St., restricted small-bodied species in the main mountain ranges and fewer Manchester, M1 5DG, UK. E-mail: [email protected] more widespread large-bodied species in the intervening lowlands. The mountain species occur in two widely separated clusters, in the Western Introduction Cape region and in the Drakensburg Mountains, but despite this are more FBA Journal System (Freshwater Biological Association) closely related to each other than to any of the lowland forms (Daniels et Freshwater crabs are a strangely neglected component of the world’s al. 2002b). These results imply that the generally small size of high altitude inland aquatic ecosystems. Despite their wide distribution throughout the species throughout Africa is not simply a convergent adaptation to the provided by tropical and warm temperate zones of the world, and their great diversity, habitat, but evidence of ancestral relationships. This conclusion is their role in the ecology of freshwaters is very poorly understood. This is supported by the recent genetic sequencing of a single individual from a nowhere more true than in Africa, where crabs occur in almost every mountain stream in Tanzania that showed it to be more closely related to freshwater system, yet even fundamentals such as their higher taxonomy mountain species than to riverine species in South Africa (S. -

Ecomorphology of Feeding in Coral Reef Fishes
Ecomorphology of Feeding in Coral Reef Fishes Peter C. Wainwright David R. Bellwood Center for Population Biology Centre for Coral Reef Biodiversity University of California at Davis School of Marine Biology and Aquaculture Davis, California 95616 James Cook University Townsville, Queensland 4811, Australia I. Introduction fish biology. We have attempted to identify generalities, II. How Does Morphology Influence Ecology? the major patterns that seem to cut across phylogenetic III. The Biomechanical Basis of Feeding Performance and geographic boundaries. We begin by constructing IV. Ecological Consequences of Functional a rationale for how functional morphology can be used Morphology to enhance our insight into some long-standing eco- V. Prospectus logical questions. We then review the fundamental me- chanical issues associated with feeding in fishes, and the basic design features of the head that are involved in prey capture and prey processing. This sets the stage I. Introduction for a discussion of how the mechanical properties of fish feeding systems have been modified during reef fish di- nce an observer gets past the stunning coloration, versification. With this background, we consider some O surely no feature inspires wonder in coral reef of the major conclusions that have been drawn from fishes so much as their morphological diversity. From studies of reef fish feeding ecomorphology. Because of large-mouthed groupers, to beaked parrotfish, barbeled space constraints we discuss only briefly the role of goatfish, long-snouted trumpet fish, snaggle-toothed sensory modalities--vision, olfaction, electroreception, tusk fish, tube-mouthed planktivores, and fat-lipped and hearingmbut these are also significant and diverse sweet lips, coral reef fishes display a dazzling array elements of the feeding arsenal of coral reef fishes and of feeding structures. -

Species Biological Report Neosho Mucket (Lampsilis Rafinesqueana)
Species Biological Report Neosho Mucket (Lampsilis rafinesqueana) Cover photo: Dr. Chris Barnhart (Missouri State University) Prepared by: The Neosho Mucket Recovery Team This species biological report informs the Draft Recovery Plan for the Neosho Mucket (Lampsilis rafinesqueana) (U.S. Fish and Wildlife Service 2017). The Species Biological Report is a comprehensive biological status review by the U.S. Fish and Wildlife Service (Service) for the Neosho Mucket and provides an account of species overall viability. A Recovery Implementation Strategy, which provides the expanded narrative for the recovery activities and the implementation schedule, is available at https://www.fws.gov/arkansas-es/. The Recovery Implementation Strategy and Species Biological Report are finalized separately from the Recovery Plan and will be updated on a routine basis. Executive Summary The Neosho Mucket is a freshwater mussel endemic to the Illinois, Neosho, and Verdigris River basins in Arkansas, Kansas, Missouri, and Oklahoma. It is associated with shallow riffles and runs comprising gravel substrate and moderate to swift currents, but prefers near-shore areas or areas out of the main current in Shoal Creek and Illinois River. It does not occur in reservoirs lacking riverine characteristics. The life-history traits and habitat requirements of the Neosho Mucket make it extremely susceptible to environmental change (e.g., droughts, sedimentation, chemical contaminants). Mechanisms leading to the decline of Neosho Mucket range from local (e.g., riparian clearing, chemical contaminants, etc.), to regional influences (e.g., altered flow regimes, channelization, etc.), to global climate change. The synergistic (interaction of two or more components) effects of threats are often complex in aquatic environments, making it difficult to predict changes in mussel and fish host(s) distribution, abundance, and habitat availability that may result from these effects. -

Facilitating Foundation Species: the Potential for Plant–Bivalve Interactions to Improve Habitat Restoration Success
Received: 28 March 2019 | Accepted: 3 February 2020 DOI: 10.1111/1365-2664.13605 REVIEW Facilitating foundation species: The potential for plant–bivalve interactions to improve habitat restoration success Karine Gagnon1 | Eli Rinde2 | Elizabeth G. T. Bengil3,4 | Laura Carugati5 | Marjolijn J. A. Christianen6,7 | Roberto Danovaro5,8 | Cristina Gambi5 | Laura L. Govers7,9 | Silvija Kipson10 | Lukas Meysick1 | Liina Pajusalu11 | İnci Tüney Kızılkaya3,12 | Johan van de Koppel9,13 | Tjisse van der Heide7,9,14 | Marieke M. van Katwijk7 | Christoffer Boström1 1Environmental and Marine Biology, Åbo Akademi University, Turku, Finland; 2Norwegian Institute for Water Research, Oslo, Norway; 3Mediterranean Conservation Society, Izmir, Turkey; 4Girne American University, Marine School, Girne, TRNC via Turkey; 5Department of Life and Environmental Sciences, Polytechnic University of Marche, Ancona, Italy; 6Aquatic Ecology and Water Quality Management Group, Wageningen University, Wageningen, The Netherlands; 7Department of Environmental Science, Institute for Wetland and Water Research, Radboud University Nijmegen, Nijmegen, The Netherlands; 8Stazione Zoologica Anton Dohrn, Naples, Italy; 9Groningen Institute for Evolutionary Life Sciences, University of Groningen, Groningen, The Netherlands; 10Faculty of Science, Department of Biology, University of Zagreb, Zagreb, Croatia; 11Estonian Marine Institute, University of Tartu, Tallinn, Estonia; 12Faculty of Science, Ege University, Izmir, Turkey; 13Royal Netherlands Institute for Sea Research and Utrecht -

The Interactive Effects of Predators, Resources, and Disturbance On
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-23-2010 The nI teractive Effects of Predators, Resources, and Disturbance on Freshwater Snail Populations from the Everglades Clifton B. Ruehl Florida International University, [email protected] DOI: 10.25148/etd.FI10080412 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Biology Commons, Other Ecology and Evolutionary Biology Commons, Population Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Ruehl, Clifton B., "The nI teractive Effects of Predators, Resources, and Disturbance on Freshwater Snail Populations from the Everglades" (2010). FIU Electronic Theses and Dissertations. 266. https://digitalcommons.fiu.edu/etd/266 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida THE INTERACTIVE EFFECTS OF PREDATORS, RESOURCES, AND DISTURBANCE ON FRESHWATER SNAIL POPULATIONS FROM THE EVERGLADES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BIOLOGY by Clifton Benjamin Ruehl 2010 To: Dean Kenneth Furton choose the name of dean of your college/school College of Arts and Sciences choose the name of your college/school This dissertation, written by Clifton Benjamin Ruehl, and entitled The Interactive Effects of Predators, Resources, and Disturbance on Freshwater Snail Populations from the Everglades, having been approved in respect to style and intellectual content, is referred to you for judgment. -

Page 2______The Shell-O-Gram______Vol 56 No
July - August, 2015_____________________________________________________________Volume 56 No. 4 Programs There will be no Jacksonville Shell Club (JSC) meeting this July. We hope members will be out in the field collecting and enjoying themselves in the long summer days. Let's gather some "Marine Observations" and share them at the August meeting. On Thursday, August 27, the JSC will convene at the usual time (7:00 PM) and place (Southeast Branch, Jacksonville Public Library <http://jpl.coj.net/lib/branches/se.html>). The shell-of-the-month will be presented by Harry Lee. His choice will be made at the annual Conchologists of America (COA) convention in Weston, FL (July 12-18). It will be the most spectacular shell he observes at the scientific programs, oral and silent auctions, bourse, on the field trips, and in more impromptu encounters. Rick Edwards, who is also planning to attend, will do the photography. The main program will be given by Charlotte Thorpe, who is fresh back from a diving expedition to the Dominican Republic. Likely there'll be several living mollusks caught in natural poses by Char's well-honed underwater photographic skills. President’s Message Dear JSC Members, There is not much activity to mention for the club over the next couple of months, we are currently cruising in idle mode. Summer is upon us and for all of us currently living in Florida, the past few days have already brought on that incredibly warm and humid feeling. With that said, I am dreaming of shelling on the beach for some marine snails on a warm sunny day, shelling in the bush for some land snails after a good rain, or cruising down a nice cool river or creek with my snorkeling gear looking for some aquatics! I'd probably enjoy self propelling a jet back through the breeze, but it seems there have been no flying snails discovered as of yet, so I'll pass on that adventure for the time being. -
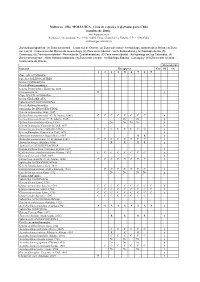
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

Molluscan Assemblages of Seagrass-Covered and Bare Intertidal Flats on the Banc D'arguin, Mauritania, in Relation to Charact
University of Groningen Molluscan assemblages of seagrass-covered and bare intertidal flats on the Banc d'Arguin, Mauritania, in relation to characteristics of sediment and organic matter Honkoop, Pieter J. C.; Berghuis, Eilke M.; Holthuijsen, Sander; Lavaleye, Marc S. S.; Piersma, Theunis Published in: Journal of Sea Research DOI: 10.1016/j.seares.2008.07.005 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2008 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Honkoop, P. J. C., Berghuis, E. M., Holthuijsen, S., Lavaleye, M. S. S., & Piersma, T. (2008). Molluscan assemblages of seagrass-covered and bare intertidal flats on the Banc d'Arguin, Mauritania, in relation to characteristics of sediment and organic matter. Journal of Sea Research, 60(4), 235-243. https://doi.org/10.1016/j.seares.2008.07.005 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. -

Present State of the Knowledge of Aquatic Mollusks in Peru
Rev. pero. biol. 6(1): 5-47 (1999) ISSN 1561-0837 © Facultad de Ciencias Biológicas UNMSM ESTADO ACTUAL DEL CONOCIMIENTO DE LOS MOLUSCOS ACUÁTICOS EN EL PERÚ PRESENT STATE OF THE KNOWLEDGE OF AQUATIC MOLLUSKS IN PERU Carlos Paredes, Pedro Huamán, Franz Cardoso, Ronald Vivar y Víctor Vera* RESUMEN Se revisa los antecedentes y el estado actual del conocimiento acerca de los moluscos acuáticos en el Perú. Los moluscos marinos y dulceacuícolas se tratan separadamente, y se da información sobre el número de familias, géneros y especies conocidas, su distribución geográfica y estado de su conserva ción y utilización por el hombre. Se incluye listas actualizadas de las especies. Palabras clave: Mo!lusca, Biodiversidad, Utilización y Conservación, Pacífico Sudeste. ABSTRACT The present state of knowiedge on the aquatic mollusks of Peru is reviewed herein. Marine and freshwater mollusks are treated separately, offering information on numbers offamilies, genera and known species, their geographical distribution, their conservation status and utilization by mano Updated species lists are included. Key words: Mollusca, Biodiversity, Utilization and conservation, southeastern Pacifico INTRODUCCiÓN Se estima que, aproximadamente, han sido descritas más de 100000 especies vivientes y El Phylum Mollusca incluye invertebrados se conoce más de 20 000 especies fósiles marinos, dulceacuÍcolas y terrestres, tan dife (Solem, 1974), y on los gasterópodos y los rentes entre sí como los chitones, caracoles, bivalvos los que presentan mayor diversidad babosas, almejas, ostras, mejillones y "conchas con 67 000 y 15 000 especies, respectivamen colmillo", así como los nautilos, calamares y te. Por otro lado, Solem (1984) estima en 60 pulpos. Sin embargo, todos ellos tienen el cuer 000 el número de especies de moluscos mari po organizado según un plan básico común, nos, 30 000 terrestres y 5 000 dulceacuÍcolas.