Molluscan Assemblages of Seagrass-Covered and Bare Intertidal Flats on the Banc D'arguin, Mauritania, in Relation to Charact
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moluscos Del Perú
Rev. Biol. Trop. 51 (Suppl. 3): 225-284, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Moluscos del Perú Rina Ramírez1, Carlos Paredes1, 2 y José Arenas3 1 Museo de Historia Natural, Universidad Nacional Mayor de San Marcos. Avenida Arenales 1256, Jesús María. Apartado 14-0434, Lima-14, Perú. 2 Laboratorio de Invertebrados Acuáticos, Facultad de Ciencias Biológicas, Universidad Nacional Mayor de San Marcos, Apartado 11-0058, Lima-11, Perú. 3 Laboratorio de Parasitología, Facultad de Ciencias Biológicas, Universidad Ricardo Palma. Av. Benavides 5400, Surco. P.O. Box 18-131. Lima, Perú. Abstract: Peru is an ecologically diverse country, with 84 life zones in the Holdridge system and 18 ecological regions (including two marine). 1910 molluscan species have been recorded. The highest number corresponds to the sea: 570 gastropods, 370 bivalves, 36 cephalopods, 34 polyplacoforans, 3 monoplacophorans, 3 scaphopods and 2 aplacophorans (total 1018 species). The most diverse families are Veneridae (57spp.), Muricidae (47spp.), Collumbellidae (40 spp.) and Tellinidae (37 spp.). Biogeographically, 56 % of marine species are Panamic, 11 % Peruvian and the rest occurs in both provinces; 73 marine species are endemic to Peru. Land molluscs include 763 species, 2.54 % of the global estimate and 38 % of the South American esti- mate. The most biodiverse families are Bulimulidae with 424 spp., Clausiliidae with 75 spp. and Systrophiidae with 55 spp. In contrast, only 129 freshwater species have been reported, 35 endemics (mainly hydrobiids with 14 spp. The paper includes an overview of biogeography, ecology, use, history of research efforts and conser- vation; as well as indication of areas and species that are in greater need of study. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

Page 2______The Shell-O-Gram______Vol 56 No
July - August, 2015_____________________________________________________________Volume 56 No. 4 Programs There will be no Jacksonville Shell Club (JSC) meeting this July. We hope members will be out in the field collecting and enjoying themselves in the long summer days. Let's gather some "Marine Observations" and share them at the August meeting. On Thursday, August 27, the JSC will convene at the usual time (7:00 PM) and place (Southeast Branch, Jacksonville Public Library <http://jpl.coj.net/lib/branches/se.html>). The shell-of-the-month will be presented by Harry Lee. His choice will be made at the annual Conchologists of America (COA) convention in Weston, FL (July 12-18). It will be the most spectacular shell he observes at the scientific programs, oral and silent auctions, bourse, on the field trips, and in more impromptu encounters. Rick Edwards, who is also planning to attend, will do the photography. The main program will be given by Charlotte Thorpe, who is fresh back from a diving expedition to the Dominican Republic. Likely there'll be several living mollusks caught in natural poses by Char's well-honed underwater photographic skills. President’s Message Dear JSC Members, There is not much activity to mention for the club over the next couple of months, we are currently cruising in idle mode. Summer is upon us and for all of us currently living in Florida, the past few days have already brought on that incredibly warm and humid feeling. With that said, I am dreaming of shelling on the beach for some marine snails on a warm sunny day, shelling in the bush for some land snails after a good rain, or cruising down a nice cool river or creek with my snorkeling gear looking for some aquatics! I'd probably enjoy self propelling a jet back through the breeze, but it seems there have been no flying snails discovered as of yet, so I'll pass on that adventure for the time being. -
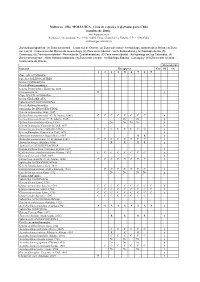
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

Present State of the Knowledge of Aquatic Mollusks in Peru
Rev. pero. biol. 6(1): 5-47 (1999) ISSN 1561-0837 © Facultad de Ciencias Biológicas UNMSM ESTADO ACTUAL DEL CONOCIMIENTO DE LOS MOLUSCOS ACUÁTICOS EN EL PERÚ PRESENT STATE OF THE KNOWLEDGE OF AQUATIC MOLLUSKS IN PERU Carlos Paredes, Pedro Huamán, Franz Cardoso, Ronald Vivar y Víctor Vera* RESUMEN Se revisa los antecedentes y el estado actual del conocimiento acerca de los moluscos acuáticos en el Perú. Los moluscos marinos y dulceacuícolas se tratan separadamente, y se da información sobre el número de familias, géneros y especies conocidas, su distribución geográfica y estado de su conserva ción y utilización por el hombre. Se incluye listas actualizadas de las especies. Palabras clave: Mo!lusca, Biodiversidad, Utilización y Conservación, Pacífico Sudeste. ABSTRACT The present state of knowiedge on the aquatic mollusks of Peru is reviewed herein. Marine and freshwater mollusks are treated separately, offering information on numbers offamilies, genera and known species, their geographical distribution, their conservation status and utilization by mano Updated species lists are included. Key words: Mollusca, Biodiversity, Utilization and conservation, southeastern Pacifico INTRODUCCiÓN Se estima que, aproximadamente, han sido descritas más de 100000 especies vivientes y El Phylum Mollusca incluye invertebrados se conoce más de 20 000 especies fósiles marinos, dulceacuÍcolas y terrestres, tan dife (Solem, 1974), y on los gasterópodos y los rentes entre sí como los chitones, caracoles, bivalvos los que presentan mayor diversidad babosas, almejas, ostras, mejillones y "conchas con 67 000 y 15 000 especies, respectivamen colmillo", así como los nautilos, calamares y te. Por otro lado, Solem (1984) estima en 60 pulpos. Sin embargo, todos ellos tienen el cuer 000 el número de especies de moluscos mari po organizado según un plan básico común, nos, 30 000 terrestres y 5 000 dulceacuÍcolas. -

Marginellidae
WMSDB - Worldwide Mollusc Species Data Base Family: MARGINELLIDAE Author: Claudio Galli - [email protected] (updated 07/set/2015) Class: GASTROPODA --- Clade: CAENOGASTROPODA-HYPSOGASTROPODA-NEOGASTROPODA-MURICOIDEA ------ Family: MARGINELLIDAE Fleming, 1828 (Sea) - Alphabetic order - when first name is in bold the species has images Taxa=1910, Genus=38, Subgenus=1, Species=1064, Subspecies=25, Synonyms=781, Images=683 abbreviata , Volvarina abbreviata (C.B. Adams, 1850) abbreviata , Marginella abbreviata C.B. Adams, 1850 - syn of: Volvarina abbreviata (C.B. Adams, 1850) abbreviata , Volvarina abbreviata C.B. Adams, 1850 - syn of: Volvarina abbreviata (C.B. Adams, 1850) abbreviatum , Prunum abbreviatum C.B. Adams, 1850 - syn of: Volvarina abbreviata (C.B. Adams, 1850) abreviata , Volvarina abreviata S.A.A. Petit De La Saussaye, 1851 - syn of: Volvarina abbreviata (C.B. Adams, 1850) abyssicola , Marginella abyssicola T.A. de M. Monterosato in É.A.A. Locard, 1897 - syn of: Gibberula abyssicola É.A.A. Locard, 1897 abyssinebulosa , Marginella nebulosa abyssinebulosa W. Massier, 2004 abyssorum, Prunum abyssorum (J.R. le B. Tomlin, 1916) accola , Marginella accola B. Roth & E.V. Coan, 1968 - syn of: Persicula accola B. Roth & E.V. Coan, 1968 aciesa , Eratoidea aciesa T. McCleery, 2011 acuta , Eratoidea acuta T. McCleery, 2011 acutulla , Eratoidea acutulla T. McCleery, 2011 adamasoides , Marginella adamasoides M. Lussi, 2013 adamkusi , Marginella adamkusi L. Bozzetti, 1994 adamsoides, Marginella adamsoides M. Lussi, 2013 adansoni , Glabella adansoni (L.C. Kiener, 1834) adela , Volvarina adela (K.H.J. Thiele, 1925) adelum , Prunum adelum K.H.J. Thiele, 1925 - syn of: Volvarina adela (K.H.J. Thiele, 1925) adrianadiae , Volvarina adrianadiae T. Cossignani, 2006 aequinoctialis , Marginella aequinoctialis F. -
Publicaciones Especiales Instituto De Ciencias Del Mar Y Limnología
PUBLICACIONES ESPECIALES INSTITUTO DE CIENCIAS DEL MAR Y LIMNOLOGÍA MOLUSCOS DE UN SISTEMA LAGUNAR TROPICAL EN EL SUR DEL GOLFO DE MÉXICO (LAGUNA DE TÉRMINOS, CAMPECHE) MOLLUSKS OF A TROPICAL LAGOON SYSTEM IN THE SOUTHERN GULF OF MEXICO (LAGUNA DE TÉRMINOS, CAMPECHE) ANTONIO GARCÍA-CUBAS Universidad Nacional Autónoma de México. Instituto de Ciencias del Mar y Limnología. Laboratorio de Malacología. Contribución 294 del Instituto de Ciencias del Mar y Limnología, UNAM. RESUMEN Este estudio es el primer trabajo integrado que se lleva a cabo en México, acerca de los moluscos de sistemas ecológicos costeros del Golfo de México; sintetizando aspectos morfológicos, sistemáticos, ecológicos y zoogeográficos de este grupo faunístico en la Laguna de Términos, Campeche. Se encuentran en el área, por lo menos 173 especies, 95 gasterópodos, 74 bivalvos, 2 cefalópodos y un poliplacóforo. Estas especies se presentan con una frecuencia y variada distribución junto al gradiente de diversidad, correlacionados con la salinidad, sustrato y vegetación sumergida, permiten caracterizar cuatro conjuntos faunísticos: a) Lagunas interiores asociadas a los ríos, b) Lagunas interiores que desembocan a la Laguna de Términos, c) Cuenca lagunar principal y d) Áreas de influencia marina. Las especies que revisten importancia comercial son: Rangia cuneata, Rangia flexuosa y Polymesoda caroliniana. (En las lagunas interiores asociadas a los ríos; Crassostrea virginica. (En las lagunas interiores que desembocan en la Laguna de Términos); Crassostrea rizophorae, Melongena melongena, Pleuroploca gigantea y Strombus alatus. (En áreas de influencia marina). En este estudio se amplía el rango de distribución de 13 especies, 8 bivalvos y 7 gasterópodos; cuyos resultados muestran que los límites de las Provincias Caribeana y Carolineana en su confluencia con la Texana son poco definidos. -

Seasonal Changes in Mollusc Abundance in a Tropical Intertidal Ecosystem, Banc D'arguin
Estuarine, Coastal and Shelf Science 136 (2014) 26e34 Contents lists available at ScienceDirect Estuarine, Coastal and Shelf Science journal homepage: www.elsevier.com/locate/ecss Seasonal changes in mollusc abundance in a tropical intertidal ecosystem, Banc d’Arguin (Mauritania): Testing the ‘depletion by shorebirds’ hypothesisq,qq Mohamed Vall Ahmedou Salem a, Matthijs van der Geest b, Theunis Piersma b,c, Younès Saoud a, Jan A. van Gils b,* a Laboratoire de Biologie Appliquée et Pathologie, Département de Biologie, Faculté des Science, B.P. 2121, Tetouan, Morocco b Royal Netherlands Institute for Sea Research (NIOZ), P.O. Box 59, 1790 AB Den Burg, Texel, The Netherlands c Animal Ecology Group, Centre for Ecological and Evolutionary Studies, University of Groningen, P.O. Box 11103, 9700 CC Groningen, The Netherlands article info abstract Article history: At temperate latitudes densities and biomass of intertidal molluscs tend to be strongly seasonal. Here we Received 15 March 2013 provide a comparative study on seasonality of bivalves and gastropods in the tropical intertidal seagrass- Accepted 9 November 2013 covered soft sediment environment of Banc d’Arguin, Mauritania (20N, 16W). In this system, ben- Available online 18 November 2013 thivorous shorebirds exert considerable predation pressure with strong seasonal variations. It has been proposed that during the period when (adult) shorebirds are absent (MayeAugust) benthic biomass Keywords: would be able to recover, but a first test was inconclusive. Over a full year (March 2011eFebruary 2012), Banc d’Arguin each month we sampled benthic invertebrates at sixteen permanent sites. The total of 3763 specimens depletion molluscs comprised 20 species, representing eight orders and 19 families. -

Mollusques Marins De Polynésie Française
Revue d’Ecologie (Terre et Vie), Vol. 72 (3), 2017 : 215-257 BIOGÉOGRAPHIE DES MOLLUSQUES MARINS DE POLYNÉSIE FRANÇAISE 1* 2 Bernard SALVAT & Jean TRÖNDLÉ 1 PSL ‒ EPHE-CNRS-UPVD USR 3278, CRIOBE, Laboratoire d’Excellence « Corail », Université de Perpignan, Av. Paul Alduy - 66860 Perpignan cedex, France. E-mail: [email protected] 2 17 Rue Girounet, 24130 La Force, France. Correspondant du Muséum national d’Histoire naturelle de Paris, Consultant CRIOBE. E-mail: [email protected] * Auteur correspondant SUMMARY.— Biogeography of French Polynesia marine molluscs.— The distribution of marine molluscs in each of the five archipelagos composing French Polynesia is presented with reference to 2053 species perfectly identified to the specific rank. The progress of knowledge on the distribution of molluscs and the limits of the inventory presented are discussed in relation to the reality of biodiversity. The species richness by archipelago is established and shows a degree of impoverishment along a longitudinal axis, from the Society to the Tuamotu and the Gambier, and along a latitudinal axis from north to south, from the Marquesas to the Society and the Austral. The distribution of species of marine molluscs allows to establish the species specific to each archipelago and those that are common to two or more archipelagos, which together determine the affinities between the archipelagos. The Marquesas and the Austral are very original in comparison with the Society, while the Tuamotu and even the Gambier are only impoverished faunas of the Society. The endemism in French Polynesia is 11.8 % (243 endemics out of 2053 species identified). Beyond this regional rate we can precise by archipelago two levels of endemism: that which is strict for the species whose distribution is limited to this archipelago and that which includes all the endemics, the strict ones and those present in at least one other archipelago: Marquesas (9.3 and 13.6 %) - Austral (6.8 and 12 %) - Society (2 and 6.5 %) - Tuamotu (2.3 and 7.9 %) - Gambier , 7 and 4.8 %). -

FMRI TR-3 Text
ISSN 1092-194X FLORIDA MARINE RESEARCH INSTITUTE TECHNICALTECHNICAL REPORTSREPORTS Checklists of Selected Shallow-Water Marine Invertebrates of Florida David K. Camp, William G. Lyons, and Thomas H. Perkins Florida Department of Environmental Protection FMRI Technical Report TR-3 1998 Lawton Chiles Governor of Florida Florida Department of Environmental Protection Virginia B. Wetherell Secretary The Florida Marine Research Institute (FMRI) is a bureau of the Florida Department of Envi- ronmental Protection (FDEP).The FDEP’s mission is to “protect,conserve, and manage Florida’s environment and natural resources.” The FMRI conducts applied research pertinent to manag- ing marine-fishery resources and marine species of special concern in Florida. Programs at the FMRI focus on resource-management topics such as managing gamefish and shellfish populations, restoring depleted fish stocks and the habitats that support them, pro- tecting coral reefs, preventing and mitigating oil-spill damage, protecting endangered and threatened species, and managing coastal-resource information. The FMRI publishes three series: Memoirs of the Hourglass Cruises, Florida Marine Research Publi- cations, and FMRI Technical Reports. FMRI Technical Reports contain information relevant to imme- diate resource-management needs. Kenneth D. Haddad, Chief of Research Institute Editors Theresa M. Bert, David K. Camp, Paul R. Carlson, Mark M. Leiby, William G. Lyons, Anne B. Meylan, Robert G. Muller, Ruth O. Reese, Carmelo R. Tomas James F. Quinn, Jr., Science Editor Judith G. Leiby, Copy Editor Llyn C. French, Art Editor Checklists of Selected Shallow-Water Marine Invertebrates of Florida David K. Camp William G. Lyons Thomas H. Perkins Florida Department of Environmental Protection Florida Marine Research Institute 100 Eighth Avenue Southeast St. -

Molluscan Assemblages of Seagrass-Covered and Bare
University of Groningen Molluscan assemblages of seagrass-covered and bare intertidal flats on the Banc d'Arguin, Mauritania, in relation to characteristics of sediment and organic matter Honkoop, Pieter J. C.; Berghuis, Eilke M.; Holthuijsen, Sander; Lavaleye, Marc S. S.; Piersma, Theunis Published in: Journal of Sea Research DOI: 10.1016/j.seares.2008.07.005 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2008 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Honkoop, P. J. C., Berghuis, E. M., Holthuijsen, S., Lavaleye, M. S. S., & Piersma, T. (2008). Molluscan assemblages of seagrass-covered and bare intertidal flats on the Banc d'Arguin, Mauritania, in relation to characteristics of sediment and organic matter. Journal of Sea Research, 60(4), 235-243. https://doi.org/10.1016/j.seares.2008.07.005 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. -

University of Groningen Multi-Trophic Interactions Within the Seagrass Beds of Banc D'arguin, Mauritania Van Der Geest, Matthijs
University of Groningen Multi-trophic interactions within the seagrass beds of Banc d'Arguin, Mauritania van der Geest, Matthijs IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Early version, also known as pre-print Publication date: 2013 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): van der Geest, M. (2013). Multi-trophic interactions within the seagrass beds of Banc d'Arguin, Mauritania: A chemosynthesis-based intertidal ecosystem. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 25-09-2021 Multi-trophic interactions within the seagrass beds of Banc d'Arguin, Mauritania A chemosynthesis-based intertidal ecosystem Matthijs van der Geest Multi-trophic interactions within the seagrass beds of Banc d’Arguin, Mauritania A chemosynthesis-based intertidal ecosystem The work presented in this thesis was conducted at the Department of Marine Ecology (MEE), Royal Netherlands Institute for Sea Research (NIOZ) according to the requirements of the Graduate School of Science (Faculty of Mathematics and Natural Sciencees, University of Groningen).