S. Vadivel Dr.P.H.Anand, M.Sc.,M.Phil.,Ph.D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide to 275 SIVA STHALAMS Glorified by Thevaram Hymns (Pathigams) of Nayanmars
Guide to 275 SIVA STHALAMS Glorified by Thevaram Hymns (Pathigams) of Nayanmars -****- by Tamarapu Sampath Kumaran About the Author: Mr T Sampath Kumaran is a freelance writer. He regularly contributes articles on Management, Business, Ancient Temples and Temple Architecture to many leading Dailies and Magazines. His articles for the young is very popular in “The Young World section” of THE HINDU. He was associated in the production of two Documentary films on Nava Tirupathi Temples, and Tirukkurungudi Temple in Tamilnadu. His book on “The Path of Ramanuja”, and “The Guide to 108 Divya Desams” in book form on the CD, has been well received in the religious circle. Preface: Tirth Yatras or pilgrimages have been an integral part of Hinduism. Pilgrimages are considered quite important by the ritualistic followers of Sanathana dharma. There are a few centers of sacredness, which are held at high esteem by the ardent devotees who dream to travel and worship God in these holy places. All these holy sites have some mythological significance attached to them. When people go to a temple, they say they go for Darsan – of the image of the presiding deity. The pinnacle act of Hindu worship is to stand in the presence of the deity and to look upon the image so as to see and be seen by the deity and to gain the blessings. There are thousands of Siva sthalams- pilgrimage sites - renowned for their divine images. And it is for the Darsan of these divine images as well the pilgrimage places themselves - which are believed to be the natural places where Gods have dwelled - the pilgrimage is made. -

Irrigation Facilities at Feasible Locations and Modernising, Improving and Rehabilitating the Existing Irrigation Infrastructure Assumes Great Importance
PUBLIC WORKS DEPARTMENT WATER RESOURCES DEPARTMENT PERFORMANCE BUDGET 2015-2016 © Government of Tamil Nadu 2016 PUBLIC WORKS DEPARTMENT WATER RESOURCES DEPARTMENT 1.0. General Management of water resources is vital to the holistic development of the State due to the growing drinking water needs and industrialisation, in addition to the needs of fisheries, environmental flows and community uses. Taking into account the limited availability of water and increasing demand for various uses, the need for creating new irrigation facilities at feasible locations and modernising, improving and rehabilitating the existing irrigation infrastructure assumes great importance. The Government is continuously striving to improve the service delivery of the irrigation system and to increase the productivity, through improving the water use efficiency, participation of farmers in operation and maintenance, canal automation, benchmarking studies and performance evaluation studies and building the capacity of Water Resources Department officials and farmers. In addition, the Government is taking up various schemes, viz., Rivers Inter-linking schemes, Artificial Recharge Schemes, Flood Management Programme, Coastal protection works, Restoration of Traditional water bodies, Augmenting drinking water supply, etc., to harness, develop and effectively utilise the seasonal flood flows occurring over a short period of time during monsoon. 1 2.0. Outlay and Expenditure for the year 2015-2016 The performance as against budgetary provisions for the year of 2015–2016, -

Nagapattinam District 64
COASTAL DISTRICT PROFILES OF TAMIL NADU ENVIS CENTRE Department of Environment Government of Tamil Nadu Prepared by Suganthi Devadason Marine Research Institute No, 44, Beach Road, Tuticorin -628001 Sl.No Contents Page No 1. THIRUVALLUR DISTRICT 1 2. CHENNAI DISTRICT 16 3. KANCHIPURAM DISTRICT 28 4. VILLUPURAM DISTRICT 38 5. CUDDALORE DISTRICT 50 6. NAGAPATTINAM DISTRICT 64 7. THIRUVARUR DISTRICT 83 8. THANJAVUR DISTRICT 93 9. PUDUKOTTAI DISTRICT 109 10. RAMANATHAPURAM DISTRICT 123 11. THOOTHUKUDI DISTRICT 140 12. TIRUNELVELI DISTRICT 153 13. KANYAKUMARI DISTRICT 174 THIRUVALLUR DISTRICT THIRUVALLUR DISTRICT 1. Introduction district in the South, Vellore district in the West, Bay of Bengal in the East and i) Geographical location of the district Andhra Pradesh State in the North. The district spreads over an area of about 3422 Thiruvallur district, a newly formed Sq.km. district bifurcated from the erstwhile Chengalpattu district (on 1st January ii) Administrative profile (taluks / 1997), is located in the North Eastern part of villages) Tamil Nadu between 12°15' and 13°15' North and 79°15' and 80°20' East. The The following image shows the district is surrounded by Kancheepuram administrative profile of the district. Tiruvallur District Map iii) Meteorological information (rainfall / ii) Agriculture and horticulture (crops climate details) cultivated) The climate of the district is moderate The main occupation of the district is agriculture and allied activities. Nearly 47% neither too hot nor too cold but humidity is of the total work force is engaged in the considerable. Both the monsoons occur and agricultural sector. Around 86% of the total in summer heat is considerably mitigated in population is in rural areas engaged in the coastal areas by sea breeze. -

The Sarasvati Mahal
Occ AS I ONAL PUBLicATION 28 India’s Best-Kept Secret: The Sarasvati Mahal by Pradeep Chakravarthy IND I A INTERNAT I ONAL CENTRE 40, MAX MUELLER MARG , NEW DELH I -110 003 TEL .: 24619431 FAX : 24627751 1 Occ AS I ONAL PUBLicATION 28 India’s Best-Kept Secret: The Sarasvati Mahal The views expressed in this publication are solely those of the author and not of the India International Centre. The Occasional Publication series is published for the India International Centre by Cmde.(Retd.) R. Datta. Designed and produced by FACET Design. Tel:91-11-24616720, 24624336. India’s Best-Kept Secret: The Sarasvati Mahal* IIC has a very special place in my heart because when I was a student in JNU in 1996-98, almost every Friday or Saturday I used to come here and listen to lectures or concerts. Those two years made me understand our heritage not just from an artistic point of view but also from a cross-disciplinary point of view, bringing my academic learning in JNU and my understanding of culture here together. Although I must confess that I had never thought when I sat here as a member of the audience that I would actually one day come up here and speak. I gave a very provocative title to this lecture about the Thanjavur Maharaja Serfoji’s Sarasvati Mahal Library partly for raising your curiosity but as I list some of its treasures, you will understand how perfectly apt it is that I should call it India’s best-kept secret. In my hands is a classic example of the proverb that asks us not to judge a book by the covers. -

Economic and Cultural History of Tamilnadu from Sangam Age to 1800 C.E
I - M.A. HISTORY Code No. 18KP1HO3 SOCIO – ECONOMIC AND CULTURAL HISTORY OF TAMILNADU FROM SANGAM AGE TO 1800 C.E. UNIT – I Sources The Literay Sources Sangam Period The consisted, of Tolkappiyam a Tamil grammar work, eight Anthologies (Ettutogai), the ten poems (Padinen kell kanakku ) the twin epics, Silappadikaram and Manimekalai and other poems. The sangam works dealt with the aharm and puram life of the people. To collect various information regarding politics, society, religion and economy of the sangam period, these works are useful. The sangam works were secular in character. Kallabhra period The religious works such as Tamil Navalar Charital,Periyapuranam and Yapperumkalam were religious oriented, they served little purpose. Pallava Period Devaram, written by Apper, simdarar and Sambandar gave references tot eh socio economic and the religious activities of the Pallava age. The religious oriented Nalayira Tivya Prabandam also provided materials to know the relation of the Pallavas with the contemporary rulers of South India. The Nandikkalambakam of Nandivarman III and Bharatavenba of Perumdevanar give a clear account of the political activities of Nandivarman III. The early pandya period Limited Tamil sources are available for the study of the early Pandyas. The Pandikkovai, the Periyapuranam, the Divya Suri Carita and the Guruparamparai throw light on the study of the Pandyas. The Chola Period The chola empire under Vijayalaya and his successors witnessed one of the progressive periods of literary and religious revival in south India The works of South Indian Vishnavism arranged by Nambi Andar Nambi provide amble information about the domination of Hindu religion in south India. -

Tamil Nadu Public Service Commission Bulletin
© [Regd. No. TN/CCN-466/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 196/2009 2017 [Price: Rs. 156.00 Paise. TAMIL NADU PUBLIC SERVICE COMMISSION BULLETIN No. 7] CHENNAI, THURSDAY, MARCH 16, 2017 Panguni 3, Thunmugi, Thiruvalluvar Aandu-2048 CONTENTS DEPARTMENTAL TESTS—RESULTS, DECEMBER 2016 Name of the Tests and Code Numbers Pages Pages Departmental Test For officers of The Co-operative Departmental Test For Members of The Tamil Nadu Department - Co-operation - First Paper (Without Ministerial Service In The National Employment Books) (Test Code No. 003) .. 627-631 Service (Without Books)(Test Code No. 006) .. 727 Departmental Test For officers of The Co-operative The Jail Test - Part I - (A) The Indian Penal Code (With Department - Co-operation - Second Paper (Without Books) (Test Code No. 136) .. .. 728-729 Books) (Test Code No. 016) .. .. 632-636 Departmental Test For officers of The Co-operative The Jail Test - Part I - (B) The Code of Criminal 729-730 Department - Auditing - First Paper (Without Procedure (With Books) (Test Code No. 154) .. Books)(Test Code No. 029) .. .. 636-641 The Jail Test - Part Ii -- Juvenile Justice (Care And Departmental Test For officers of The Co-operative Protection.. of Children) Act, 2000 (Central Act 56 of Department - Auditing - Second Paper (Without 2000).. (With Books) (Test Code No. 194) .. 730 Books)(Test Code No. 044) .. 641-645 The Jail Test -- Part I -- (C) Laws, Rules, Regulations Departmental Test For officers of The Co-operative And Orders Relating To Jail Management (With Department - Banking (Without Books) (Test Code Books)(Test Code No. 177) .. .. 731-732 No. -
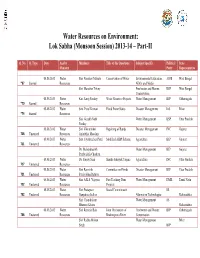
Water Resources on Environment: Lok Sabha (Monsoon Session) 2013-14 – Part-II
Water Resources on Environment: Lok Sabha (Monsoon Session) 2013-14 – Part-II Q. No. Q. Type Date Ans by Members Title of the Questions Subject Specific Political State Ministry Party Representative 08.08.2013 Water Shri Narahari Mahato Conservation of Water Environmental Education, AIFB West Bengal *67 Starred Resources NGOs and Media Shri Manohar Tirkey Freshwater and Marine RSP West Bengal Conservation 08.08.2013 Water Km. Saroj Pandey Water Resource Projects Water Management BJP Chhattisgarh *70 Starred Resources 08.08.2013 Water Smt. Putul Kumari Flood Prone States Disaster Management Ind. Bihar *74 Starred Resources Shri Gorakh Nath Water Management BSP Uttar Pradesh Pandey 08.08.2013 Water Shri Vikrambhai Repairing of Bunds Disaster Management INC Gujarat 708 Unstarred Resources Arjanbhai Maadam 08.08.2013 Water Smt. Jayshreeben Patel Modified AIBP Scheme Agriculture BJP Gujarat 711 Unstarred Resources Dr. Mahendrasinh Water Management BJP Gujarat Pruthvisinh Chauhan 08.08.2013 Water Dr. Sanjay Sinh Sharda Sahayak Yojana Agriculture INC Uttar Pradesh 717 Unstarred Resources 08.08.2013 Water Shri Ramsinh Committee on Floods Disaster Management BJP Uttar Pradesh 721 Unstarred Resources Patalyabhai Rathwa 08.08.2013 Water Shri A.K.S. Vijayan Fast Tracking Dam Water Management DMK Tamil Nadu 722 Unstarred Resources Projects 08.08.2013 Water Shri Prataprao Social Commitment SS 752 Unstarred Resources Ganpatrao Jadhav Alternative Technologies Maharashtra Shri Chandrakant Water Management SS Bhaurao Khaire Maharashtra 08.08.2013 Water -

Thanjavur Temple History in Tamil Pdf Download
Thanjavur temple history in tamil pdf download Continue Thanjai Peria Kovil History in Tamil PdfDownload Thanjai Peria Kovil History in Tamil Pdf Location in Tamil Nada, India Show Map of Tamil Nadu Basic Information Location: Festivals District State Of India Architectural Description creator Completed 1010 AD Inscriptions of Tamil and Grantha scripts Brihadishvara Temple, also referred to as Rajesvara Peruvudaiyr or Brihadeeswarar Temple, dedicated located in, India.King Raja Raja Cholan built Tanjay Grand Temple (also called Thanjavur Peria Koil, Peria Covil, Koyil, and Tanjore Grand Temple) more than a thousand years ago; it took 4 years of construction and was dedicated in 1010 AD தைச ரகவர ேகா எ, தைச ெபய ேகா ('Big.Thanjai periya periya periya kovil history Brihadeeswarar Temple. TanjoreView Temple Brihadiswarar, Marata Palace, zakova field, Rajarajan Manimandapam (Bell Tower) and Tamil University, Siwangangai ParkCoordinates: 10'47'00N79'8'10E/ 10.7833 3'N 79.13611'ECoordinates: 10'47'00N79'8'10E / 10.78333 N 79.13 611'ECountryIndiaStateMil NaduRegionChola NaduDistrictThanjavur-1uvery Delta Government Municipal Corporationur CityArea - Total 38.33 km2 (14.80 sq m) Area rank11 Rise88 m (289 ft) Population (2011) - Total 290 724 - Density7 600/km2 (20,000/sq. mi) Demonym (s)TanjoriansLanguages - OfficialTamilTime zoneUTC-5:30 (IST)PIN613 xxxTelephone code04362Vehicle registrationTN-49Thanjavur, formerly Tanjore, is an Indian city in Tamilnad. Thanjavur is an important centre of South Indian religion, art and architecture. Most of the temples of the Great Living Chola, which are UN World Heritage Sites, are located in And around Thanjavur. The most important of them, the temple of Brihadesvar, is located in the center of the city. -

Arasalar River)
Revised Action plan for restoration of polluted river stretches (ARASALAR RIVER) Arasalar River Puducherry Pollution Control Committee Department of Science, Technology & Environment Government of Puducherry REVISED ACTION PLAN FOR RESTORATION OF ARASALAR RIVER U.T. OF PUDUCHERRY ( KARAIKAL REGION) Preamble: In pursuance of the Hon’ble National Green Tribunal (Principal Bench), New Delhi, orders dt. 20.09.2018 and 19.12.2018 in original application No. 673/2018 in the matter of News item published in “The Hindu” Titled more river stretches are now critically polluted - Central Pollution Control Board. Action plans were framed with the objective of restoration of Arasalar river , Karaikal to meet the bathing standards of pH, Dissolved Oxygen (DO), Biological Oxygen Demand (BOD), Faecal coliforms and Faecal Streptococci within 2 years period. River Rejuvenation committee has been constituted vide OM No. 4739/PPCC/RRC/SCI- I/2018 dt. 13.11.2018 to prepare and execute the action plan. A meeting was held on 28.01.2019 under the Chairmanship of Hon’ble Chief Minister on preparation of revised Action plan to restore polluted river stretches. Fig.1 Revised Action plan presented before the Hon’ble Chief Minister of Puducherry In compliance with the Hon’ble NGT order dated 06.12.2019, State Level Monitoring Committee (SLMC) has been constituted under the Chairmanship of Secretary (Envt) vide order no. 6836/PPCC/NGT/SEE/2020 dt. 08.01.2020. First State Level Monitoring Meeting was held on 29.01.2020. 2 Arasalar: Arasalar River is having a total run of 24 Km, enters Karaikal, a little east of kalanganni. -

The Journal of the Music Academy Devoted to the Advancement of the Science and Art of Music
ISSN. 0970 -3101 THE JOURNAL OF THE MUSIC ACADEMY DEVOTED TO THE ADVANCEMENT OF THE SCIENCE AND ART OF MUSIC Vol. LXVI 1995 »T5CtCT w qrafor w fagffa w * II "/ dwell not in Vaikuntha, nor in the hearts o f Yogins nor in the Sun; (but) where my bhaktas sing, there be I, NaradaV' Edited by T.S. PARTHASARATHY The Music Academy, Madras 306, T.T.K. Road, Madras - 600 014. Annual Subscript sign $ 3-00 THE JOURNAL OF THE MUSIC ACADEMY DEVOTED TO THE ADVANCEMENT OF THE SCIENCE AND ART OF MUSIC Vol. LXVI 1995 t TOTfiT 1 V f t I *nr nurfer w ftgrfir h r * n "I dwell not in Vaikuntha, nor in the hearts o f Yogins nor in the Sun; (but) where my bhaktas sing, there be I, Narada!" Edited by T.S. PARTHASARATHY The Music Academy, Madras 306, T.T.K. Road, Madras - 600 014. Annual Subscription - Inland oreign $ 3-00 r OURSELVES This Journal is published as an Annual. All correspondence relating to the Journal should be sent to The Editor Journal of the Music Academy, 306, T.T.K. Road, Madras - 600 014. Articles on music and dance are accepted for publication on the understanding that they are contributed solely to the Journal of the Music Academy. Manuscripts should be legibly written or, preferably, typewritten (double -spaced and on one side of the paper only) and should be signed by the writer (giving his or her address in full). The Editor of the Journal is not responsible for the views expressed by contributors in their articles. -

District Census Handbook, Thanjavur, Part X-A, Series-19
CENSUS OF INDIA, 1971 SERIES 19 TAMIL NADU PART X-A DISTRICT CENSUS HANDBOOK village and Town Directory THANJAVUR K. C HOC K A II N GAM of the Indian Administratire Service DIRECTOR OF CENSUS OPERATIONS. TAMIL NADU AND PONDICHERRY. 1972 jo Ang o 0 I I oj (/) L __________ -- o~~ ~ .., ~• II II II I .... ,: I·0 - -----_-----""- _...;.. -- CONTENTS Page No. Preface ... v Part-A VILLA GE AND TOWN DIRECTORY Introductory Note: vii-xiii (I) Village Directory ••• Amenities and land use Appendix-I Land use particulars of NOD - city urban area (Non-Municipal area) Appendix-II Abstr<lct showing Educationa), Medica1 aad other amenities available in Taluks. Alphabetical Jist of Villages. 1. Sirkali Taluk ... 2 -13 2. Mayuram ,. 14:- 35 S. Kumbakonam ,. 36 - 54 4:. Nannilam .. 55 - 84 5. Papanasam .. ... 85 -108 6. Thanjavur .. 109-134: 7. Orthanad 135-152 " ... 153 -172 8. Mannargudi .. '" 9. Nagapattinam .. - 173-190 191-210 10. Thiruthuraipundi " 11. Pattukkottai '0 211-234 12. Peravurani .. - 235-251 13. Arantangi ,. - 252-282 iv Page No. (II) Town Directory Statement: I - Status. Growth History and FunctIOnal Category of Tov.ns. 286 - 291 II - PhysIcal Aspects and Location of Towns, 1969. 292-297 lIt - Municipal Finance, 1968- 69 298-30:1 IV - Civic and other amenitle", 1969 304-309 , , V- Medical, Educational. Recreational and Cultural Facll.t1es in Towns. 1969 310 315 .. VI - Trade. Commerce. Industry and Banking, 1969 316- 321 ,. V/I- Population by rehgion. 1971 322-327 Maps f'i!'.trict map of Thanjavur Frontispiece PRf:FAGE It has been the practice since 1951 to publish for each District a Census Hand book, contaming the Census Statistics for each village and town in the Distnct together wIth certain administrative statistics collected from the various Departments of the State Government. -

Interpretation of Soil Resources Using Remote Sensing and GIS in Thanjavur District, Tamil Nadu, India
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2011, 2 (3): 525-535 ISSN: 0976-8610 CODEN (USA): AASRFC Interpretation of soil resources using remote sensing and GIS in Thanjavur district, Tamil Nadu, India J. Punithavathi*, S. Tamilenthi and R. Baskaran Department of Industries and Earth Sciences, Tamil University, Thanjavur, TN, India ______________________________________________________________________________ ABSTRACT Mapping of physiographic units and the sediments/soils of an area can give useful information for land use planning. Remote sensing technique plays an important role in the mapping of soil, physiographic units and other land resources. In this present study, Thanjavur district, Tamil Nadu has been choosen to prepare physiographic units and soil maps by using IRS-P6 satellite imagery, (Scale 1:50,000). This study reveals that the mapping of different soil/sediment types and physiographic units can be effectively and the land suitability can be inferred. Key words: Physiographic units, Soil productivity, Crops grown, Remote sensing and GIS. ______________________________________________________________________________ INTRODUCTION In view of soil resources mapping, the study area of Thanjavur district Tamil nadu has been interpreted. The Remote sensing Technique is a major tool interpreting the land resources and that can be used for a Thanjavur area like this kind of soil mapping. The major physiographic features such as alluvial and sandy plains, undulating pediplan, upland and natural vegetation, coastal plain areas or low lying lands of the study area have been interpreted. There are many previous investigation such as Ahuja R.L[1], Kumar, Ashok and Sanjay Kumar Srivastava [3], Fabos J.G.[4], Fitz and Patrick.E.A [5], Lillesand, Keieper[6], Saha and S.K.Singh [7] and many other soil resources related of the previous investigation.