Chapter 5 Benthic Community Response to Flow and Temperature in Riffles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New South Wales Class 1 Load Carrying Vehicle Operator’S Guide
New South Wales Class 1 Load Carrying Vehicle Operator’s Guide Important: This Operator’s Guide is for three Notices separated by Part A, Part B and Part C. Please read sections carefully as separate conditions may apply. For enquiries about roads and restrictions listed in this document please contact Transport for NSW Road Access unit: [email protected] 27 October 2020 New South Wales Class 1 Load Carrying Vehicle Operator’s Guide Contents Purpose ................................................................................................................................................................... 4 Definitions ............................................................................................................................................................... 4 NSW Travel Zones .................................................................................................................................................... 5 Part A – NSW Class 1 Load Carrying Vehicles Notice ................................................................................................ 9 About the Notice ..................................................................................................................................................... 9 1: Travel Conditions ................................................................................................................................................. 9 1.1 Pilot and Escort Requirements .......................................................................................................................... -
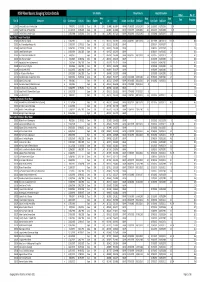
Gauging Station Index
Site Details Flow/Volume Height/Elevation NSW River Basins: Gauging Station Details Other No. of Area Data Data Site ID Sitename Cat Commence Ceased Status Owner Lat Long Datum Start Date End Date Start Date End Date Data Gaugings (km2) (Years) (Years) 1102001 Homestead Creek at Fowlers Gap C 7/08/1972 31/05/2003 Closed DWR 19.9 -31.0848 141.6974 GDA94 07/08/1972 16/12/1995 23.4 01/01/1972 01/01/1996 24 Rn 1102002 Frieslich Creek at Frieslich Dam C 21/10/1976 31/05/2003 Closed DWR 8 -31.0660 141.6690 GDA94 19/03/1977 31/05/2003 26.2 01/01/1977 01/01/2004 27 Rn 1102003 Fowlers Creek at Fowlers Gap C 13/05/1980 31/05/2003 Closed DWR 384 -31.0856 141.7131 GDA94 28/02/1992 07/12/1992 0.8 01/05/1980 01/01/1993 12.7 Basin 201: Tweed River Basin 201001 Oxley River at Eungella A 21/05/1947 Open DWR 213 -28.3537 153.2931 GDA94 03/03/1957 08/11/2010 53.7 30/12/1899 08/11/2010 110.9 Rn 388 201002 Rous River at Boat Harbour No.1 C 27/05/1947 31/07/1957 Closed DWR 124 -28.3151 153.3511 GDA94 01/05/1947 01/04/1957 9.9 48 201003 Tweed River at Braeside C 20/08/1951 31/12/1968 Closed DWR 298 -28.3960 153.3369 GDA94 01/08/1951 01/01/1969 17.4 126 201004 Tweed River at Kunghur C 14/05/1954 2/06/1982 Closed DWR 49 -28.4702 153.2547 GDA94 01/08/1954 01/07/1982 27.9 196 201005 Rous River at Boat Harbour No.3 A 3/04/1957 Open DWR 111 -28.3096 153.3360 GDA94 03/04/1957 08/11/2010 53.6 01/01/1957 01/01/2010 53 261 201006 Oxley River at Tyalgum C 5/05/1969 12/08/1982 Closed DWR 153 -28.3526 153.2245 GDA94 01/06/1969 01/09/1982 13.3 108 201007 Hopping Dick Creek -

Government Gazette of the STATE of NEW SOUTH WALES Number 98 Friday, 5 August 2005 Published Under Authoritynew by Government South Wales Advertising and Information
4063 Government Gazette OF THE STATE OF NEW SOUTH WALES Number 98 Friday, 5 August 2005 Published under authorityNew by Government South Wales Advertising and Information Public AuthoritiesLEGISLATION (Financial Arrangements) Amendment (Joint Venture Exemptions)Regulations Regulation 2005 under the Public Authorities (Financial Arrangements) Act 1987 New South Wales Her Excellency the Governor, with the advice of the Executive Council, has made the following Regulation under the Public Authorities (Financial Arrangements) Act Public1987. Authorities (Financial Arrangements) Amendment (Joint Venture Exemptions) Regulation 2005 Treasurer under the Explanatory note PublicThe object Authorities of this Regulation (Financ is to excludeial Arrangements) certain activities involving Act 1987the TAFE Commission and the Department of Education and Training from the provisions relating to joint ventures. Under Part 2D of the Act, the Treasurers’ approval is required for joint ventures entered into, or carried on, by authorities within the meaning of the Act. This Regulation is made under the Public Authorities (Financial Arrangements) Act 1987, Herincluding Excellency section 22K.the Governor, with the advice of the Executive Council, has made the following Regulation under the Public Authorities (Financial Arrangements) Act 1987. ANDREW REFSHAUGE, M.P., Treasurer Explanatory note The object of this Regulation is to exclude certain activities involving the TAFE Commission and the Department of Education and Training from the provisions relating to joint ventures. Under Part 2D of the Act, the Treasurers’ approval is required for joint ventures entered into, or carried on, by authorities within the meaning of the Act. This Regulation is made under the Public Authorities (Financial Arrangements) Act 1987, including section 22K. -

Tweed Shire Echo
THE TWEED what s www.tweedecho.com.au Volume 3 #35 new? Thursday, May 12, 2011 Advertising and news enquiries: Phone: (02) 6672 2280 [email protected] [email protected] CAB Page 12 21,000 copies every week AUDIT LOCAL & INDEPENDENT Tweed goes to P’ville shopping the dogs for the RSPCA centre plan goes off the boil Luis Feliu on the site and use the land for more housing. A shopping complex which residents Pottsville Residents Association from Pottsville and its booming Sea- president Chris Cherry this week told breeze housing estate had expected to Th e Echo that ‘the small-scale super- be built appears to be off the drawing market proposal is no more’. board altogether. ‘As Metricon could not get their Developer of Seabreeze, Metricon, full-line centre approved, they have recently backed off plans for even a now gone ahead with a residential small-scale supermarket on land it rezoning of this area and the blocks owns despite a lengthy and expensive are on sale or already sold,’ Ms Cherry battle to have a larger, full-line one said. approved there. Th e Queensland-based developer, ‘A major fl aw’ Kate McIntosh Bonnie and Sandy Oswald, Benny and Jeanette Whiteley and Fudge, Tori which has several major housing ‘As far as I am concerned this with- and Harvey Bishop are all looking forward to this Sunday’s Million Paws developments underway around drawal of promised local services to Tweed residents and their four-legged Walk for the RSPCA. Photo Jeff ‘Houndog’ Dawson Tweed Shire, now wants to use the residents who have bought in accord- friends will be pounding the pavement land for more housing. -

It's Storm Season
Are you ready? IT’S STORM SEASON Above: In both storms and floods, items not secured can end up as flying and floating debris. Plan ahead to act on time It’s officially storm season, where of 31 March 2017 – the largest seen in many pets to limit your damages and losses in the event electrical thunderstorms can roll through locations across Tweed Shire. It’s timely to of a storm or flood hitting your area. Talk your plan We want to be a resilient and ready with wind, thunder, lightning and even hail remember that this flood occurred from a through with your family and then put it on paper. community. We want to make sure on any given day. While the wettest of the relatively short storm event and a far greater And don’t forget to identify dry places where you that new residents understand the season normally comes after the New Year, flood is possible. can store belongings and clean up your yard, reality of living here. it can come anytime from October to May. The best safeguard against flood and storm is making sure that any item that can be thrown It’s more than eight months since the flood preparation. Plan now for you, your family and your around in a storm is put away or tied down. – Tumbulgum EMERGENCY CONTACTS Identify your flood trigger As a ‘rule of thumb’ for most of So, what’s your flood trigger to: During the March 2017 flood the Tweed Shire, if 300mm of rain • lift your property from any emergency, it was clear many residents falls within 24 hours you should under-storey? had little understanding of how floods expect significant flooding. -

NSW Recreational Freshwater Fishing Guide 2020-21
NSW Recreational Freshwater Fishing Guide 2020–21 www.dpi.nsw.gov.au Report illegal fishing 1800 043 536 Check out the app:FishSmart NSW DPI has created an app Some data on this site is sourced from the Bureau of Meteorology. that provides recreational fishers with 24/7 access to essential information they need to know to fish in NSW, such as: ▢ a pictorial guide of common recreational species, bag & size limits, closed seasons and fishing gear rules ▢ record and keep your own catch log and opt to have your best fish pictures selected to feature in our in-app gallery ▢ real-time maps to locate nearest FADs (Fish Aggregation Devices), artificial reefs, Recreational Fishing Havens and Marine Park Zones ▢ DPI contact for reporting illegal fishing, fish kills, ▢ local weather, tide, moon phase and barometric pressure to help choose best time to fish pest species etc. and local Fisheries Offices ▢ guides on spearfishing, fishing safely, trout fishing, regional fishing ▢ DPI Facebook news. Welcome to FishSmart! See your location in Store all your Contact Fisheries – relation to FADs, Check the bag and size See featured fishing catches in your very Report illegal Marine Park Zones, limits for popular species photos RFHs & more own Catch Log fishing & more Contents i ■ NSW Recreational Fishing Fee . 1 ■ Where do my fishing fees go? .. 3 ■ Working with fishers . 7 ■ Fish hatcheries and fish stocking . 9 ■ Responsible fishing . 11 ■ Angler access . 14 ■ Converting fish lengths to weights. 15 ■ Fishing safely/safe boating . 17 ■ Food safety . 18 ■ Knots and rigs . 20 ■ Fish identification and measurement . 27 ■ Fish bag limits, size limits and closed seasons . -

Hydrosphere Formal Report Template
Northern Rivers Regional Bulk Water Supply Strategy Draft April 2013 Disclaimer: This report has been prepared on behalf of and for the exclusive use of Northern Rivers Regional Organisation of Councils (NOROC), and is subject to and issued in accordance with the agreement between NOROC and Hydrosphere Consulting. Hydrosphere Consulting accepts no liability or responsibility whatsoever for it in respect of any use of or reliance upon this report by any third party. Copying this report without the permission of NOROC or Hydrosphere Consulting is not permitted. Suite 6, 26-54 River Street PO Box 7059, Ballina NSW 2478 Telephone: 02 6686 0006 Facsimile: 02 6686 0078 © Copyright 2013 Hydrosphere Consulting PROJECT 12-025– NOROC REGIONAL BULK WATER SUPPLY STRATEGY REV DESCRIPTION AUTHORS REVIEW APPROVAL DATE 0 Issued for Rous Water R. Campbell, K. Pratt, U. Makings, R. Campbell, M. Howland M. Howland 8/3/13 review M. Howland 1 Issued for NOROC SWMG R. Campbell, K. Pratt, M. Howland M. Howland 22/4/13 review NOROC NORTHERN RIVERS REGIONAL BULK WATER SUPPLY STRATEGY EXECUTIVE SUMMARY The Northern Rivers Regional Organisation of Councils (NOROC) has resolved to develop a long-term (50- year) regional water supply strategy in order to evaluate the potential benefits to future water supply security resulting from a regionally integrated system. This report investigates numerous interconnection and supply scenarios that aim to maximise the benefit of a regional approach and presents the key issues for consideration. E1 EXISTING WATER SUPPLIES The status of the existing water resources and current demand for water is presented in Interim Report 1 (attached as Appendix 1). -

Nsw Estuary and River Water Levels Annual Summary 2015-2016
NSW ESTUARY AND RIVER WATER LEVELS ANNUAL SUMMARY 2015–2016 Report MHL2474 November 2016 prepared for NSW Office of Environment and Heritage This page intentionally blank NSW ESTUARY AND RIVER WATER LEVELS ANNUAL SUMMARY 2015–2016 Report MHL2474 November 2016 Peter Leszczynski 110b King Street Manly Vale NSW 2093 T: 02 9949 0200 E: [email protected] W: www.mhl.nsw.gov.au Cover photograph: Coraki photo from the web camera, Richmond River Document control Issue/ Approved for issue Author Reviewer Revision Name Date Draft 21/10/2016 B Tse, MHL S Dakin, MHL A Joyner 26/10/2016 Final 04/11/2016 M Fitzhenry, OEH A Joyner 04/11/2016 © Crown in right of NSW through the Department of Finance, Services and Innovation 2016 The data contained in this report is licensed under a Creative Commons Attribution 4.0 licence. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0 Manly Hydraulics Laboratory and the NSW Office of Environment and Heritage permit this material to be reproduced, for educational or non-commercial use, in whole or in part, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. While this report has been formulated with all due care, the State of New South Wales does not warrant or represent that the report is free from errors or omissions, or that it is exhaustive. The State of NSW disclaims, to the extent permitted by law, all warranties, representations or endorsements, express or implied, with regard to the report including but not limited to, all implied warranties of merchantability, fitness for a particular purpose, or non-infringement. -

Open Space Strategy 2019-2029
Open Space Strategy 2019 – 2029 Living and loving the Tweed Contents A NEW OPEN SPACE STRATEGY ....................................................................................................................................................5 Introduction .....................................................................................................................................................................................6 Building on our success ...................................................................................................................................................................7 Defining open space ........................................................................................................................................................................8 Our open spaces ..............................................................................................................................................................................9 Benefits of open spaces................................................................................................................................................................. 11 Purpose of the Open Space Strategy.............................................................................................................................................. 12 Strategic context and relevant legislation and policy ...................................................................................................................... 13 Methodology -

Tugun Bypass Environmental Impact Statement Technical Paper Number 7 Flooding and Hydrological Assessment
Tugun Bypass Environmental Impact Statement Technical Paper Number 7 Flooding and Hydrological Assessment Tugun Bypass Alliance Offices Client: Queensland Department of Main Roads Brisbane Prepared By: WBM Oceanics Australia Denver Karratha Melbourne Prepared For: Parsons Brinckerhoff Morwell (formerly PPK Environment & Infrastructure) Newcastle Sydney Vancouver July, 2003 OCEANICS AUSTRALIA November 2004 2134070A-PR049Car- R.B13010.001.05 Tugun Bypass Environmental Impact Statement Technical Paper Number 7 Flooding and Hydrological Assessment Contents Page Number Glossary I 1. Introduction 1-1 1.1Summary of the Technical Paper 1-1 1.2Reporting of Study Findings in the EIS 1-2 2. Purpose and Approach 2-1 2.1General 2-1 2.2Design Criteria and Required Flood Immunity 2-1 3. Existing Flooding Conditions 3-1 3.1Local/Minor Catchments 3-1 3.1.1 Chainage 00 to 2,300 3-1 3.1.2 Chainage 2,800 3-1 3.1.3 Chainage 3,200 3-7 3.1.4 Chainage 3,900 3-7 3.1.5 Chainage 4,900 3-7 3.1.6 Chainage 5,450 to 6,000 3-7 3.2Tweed River Floodplain 3-9 3.2.1 Previous Studies by WBM on Tweed River 3-9 3.2.2 Tweed River Flood Model 3-9 3.2.3 Tweed River Flooding Characteristics 3-9 3.2.4 Peak Flood Levels 3-13 3.2.5 Influence of Elevated Ocean Levels 3-13 3.2.6 Peak Flood Levels for Rare Flood Events 3-15 3.2.7 Summary of Frequency of Inundation of Study Area 3-16 3.2.8 Inundation Times 3-16 3.3Coolangatta Creek 3-17 4. -

Speed Limits NSW Waters (Including Controlled Victorian Waters of Lakes Hume and Mulwala)
Speed Limits NSW Waters (including controlled Victorian Waters of Lakes Hume and Mulwala) In pursuance of the provisions of Section 11 of the Marine Safety Act 1998 and the New South Wales and Victorian Acts both entitled Marine Safety Legislation (Lakes Hume and Mulwala) Act 2001 Coastal waterways listed from south to north on pages 2-34 Inland waterways listed alphabetically on pages 35-43 March 2019 rms.nsw.gov.au 1 COASTAL WATERS GENERALLY SOUTH TO NORTH BMap 14A Wonboyn River (Entrance) Area – The navigable waters of that part of Wonboyn River between lines across the waterway, firstly in the east commencing from a point on the north western extremity of the point known locally as Dollys Island at the entrance to Wonboyn River with the Tasman Sea in a generally easterly direction across the lake entrance area to a point on the opposite northern shore of North Wonboyn Beach and secondly in the west from a point on the south eastern extremity of the point known locally as Round Hill in a southerly direction approximately four hundred (400) metres to a point directly opposite on the southern shore of Nadgee Nature Reserve - four knots. BMap 14A Twofold Bay - (Quarantine Bay) Area - The navigable waters of Quarantine Bay enclosed by an imaginary line from the western extremity of the breakwater to the south-eastern extremity of Quondoa Point - four knots. BMap 14A Curalo Lagoon Area – The navigable waters of the whole of Curalo Lagoon and its tributaries upstream from its entrance with the Tasman Sea – four knots. BMap 14A Merimbula -

National Class 1 Agricultural Vehicle and Combination Mass and Dimension Exemption Notice Operator’S Guide
National Class 1 Agricultural Vehicle and Combination Mass and Dimension Exemption Notice Operator’s Guide November 2020 Contents Introduction and preliminary information 3 Special conditions for travel in New South Wales 24 Agricultural vehicles and combinations 6 Special conditions for travel in Queensland 34 Dimension and mass limits 8 Special conditions for travel in South Australia 42 Braking and tow mass ratio requirements 11 Special conditions for travel in Victoria 43 Approved networks and mapped conditions 12 Appendix 1 – New South Wales Agricultural Vehicle Route Assessment 44 Dimension and pilot conditions for allowable night travel 14 Appendix 2 – Sugarcane harvester excluded areas Travel conditions 15 and approved roads 46 Warning device conditions 16 Appendix 3 – Sample list of common agricultural vehicle Pilot vehicles 18 conditions from Schedule 8 of the MDL Regulation 47 Escort vehicle requirements 21 Appendix 4 – Portable road side warning sign designs Special conditions for eligible cotton harvesters 22 for Queensland 52 Special conditions for travel in the Australian Capital Appendix 5 – Road Manager conditions 57 Territory 23 Appendix 6 – Braking performance test 58 2 National Class 1 Agricultural Vehicle and Combination Mass and Dimension Exemption Notice Operator’s Guide Introduction and preliminary information Purpose If travel is not allowed under the Notice This National Class 1 Agricultural Vehicle and Combination If travel is not allowed under the Notice, access may be Mass and Dimension Exemption Notice Operator’s Guide (the possible under a Class 1 mass and/or dimension permit for Guide) complements the National Class 1 Agricultural Vehicle the agricultural vehicle or combination, subject to a granting of and Combination Mass and Dimension Exemption Notice consent by the relevant road manager/s.