AIA News 140 Spring 2007
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Terminologies
FISH TERMINOLOGIES Monument Type Thesaurus Report Format: Hierarchical listing - class Notes: Classification of monument type records by function. -

Spring Newsletter 2021
THE LEICESTERSHIRE INDUSTRIAL HISTORY SOCIETY Founded in 1969 www.lihs.org.uk Newsletter Volume 8 No 1 Spring 2021 In this Issue…. Page 9 Page 4 The Great Central Railway Station. In memory of Dennis Calow 1926-2020 Page 20 Page 16 Page 23 Leicestershire Industrial History Society Spring 2021 The Leicestershire Industrial History Society Founded in 1969 Contents 3 A View from the Chair 4 The Corah Factory - Our Built Heritage vs. the Developers 6 A Tale of Two Tunnels 10 The Samuel Street Hydraulic Power Station and Tower 16 The Impact of a Railway on a Developed Urban Area - the GCR (Adapted from a 1984 article by Peter Neaverson) 20 A Tale of Two Tunnels (contd.) 22 Ullesthorpe Windmill 23 New Light on the Sheepy Magna Wheelwright’s Workshop 25 Leicestershire Railway Tunnels - Update 26 Thomas Cook, the first Railway Excursion Revisited 27 The Dale Abbey Furnace 2 Leicestershire Industrial History Society Spring 2021 A View from the Chair out by Peter Neaverson in 1984 on the impact Chris Hossack on the urban environment with the arrival of the GCR in Leicester. Last Autumn we suggested that we would With the Thomas Cook archive now at ROLLR produce four issues of the newsletter this year and hopes of having a lecture soon on the in order to better stay in touch with our subject once the huge amount of material has membership. However, Bill Pemberton, been sifted through, an interesting twist to the treasurer, membership secretary and Zoom tale is revealed as to what constituted the first master in chief has been producing frequent railway excursion. -

Aa Box ABATTOIR ABBEY Abbey Barn Abbey Barn Abbey Bridge Abbey Bridge Abbey Church Abbey Church Abbey Gate Abbey Gate Abbey Gate
Aa Box Abbey Bridge USE : MOTORING TELEPHONE BOX USE : ABBEY ABATTOIR Abbey Bridge UF : Slaughter House USE : BRIDGE UF : Butching House BT : FOOD PROCESSING SITE Abbey Church RT : BUTCHERY SITE USE : ABBEY RT : SHAMBLES RT : SMOKE HOUSE Abbey Church RT : GLUE FACTORY USE : CHURCH RT : TANNERY RT : HORSEHAIR FACTORY Abbey Gate SN : A building where animals are slaughtered. USE : ABBEY ABBEY Abbey Gate UF : Benedictine Abbey UF : Arrouiasian Abbey USE : GATE UF : Augustinian Abbey UF : Victorine Abbey Abbey Gatehouse UF : Tironian Abbey USE : GATEHOUSE UF : Savigniac Abbey UF : Premonstratensian Abbey Abbey Gatehouse UF : Franciscan Abbey USE : ABBEY UF : Cistercian Abbey UF : Cluniac Abbey Abbey Kitchen UF : Bridgettine Abbey USE : ABBEY UF : Convent Chapel UF : Abbey Barn Abbey Kitchen UF : Abbey Bridge USE : KITCHEN UF : Abbey Church UF : Abbey Gate Abbey Wall UF : Abbey Gatehouse USE : PRECINCT WALL UF : Abbey Kitchen UF : Independent Abbey UF : Tironensian Abbey Abbots House UF : Conventual Chapel USE : MONASTIC DWELLING UF : Conventual Church UF : Farmery Abbots Lodging BT : RELIGIOUS HOUSE USE : MONASTIC DWELLING RT : ALMONRY RT : GUEST HOUSE ABBOTS PALACE RT : KITCHEN BT : PALACE RT : CHAPTER HOUSE SN : The official residence of an abbot. RT : CATHEDRAL RT : PRECINCT WALL ABBOTS SUMMER PALACE RT : DOUBLE HOUSE BT : PALACE RT : FRIARY RT : BISHOPS SUMMER PALACE RT : MONASTERY SN : An official residence of an abbot during the summer RT : NUNNERY months. RT : PRECEPTORY RT : PRIORY ABLUTIONS BLOCK RT : GATEHOUSE BT : DOMESTIC MILITARY BUILDING RT : REFECTORY BT : WASHING PLACE RT : CONVENT SCHOOL SN : A building housing washing facilities and toilets. The RT : CURFEW BELL TOWER term occurs mainly in a military context. -

Food8. I T Is Only Within the Last Ten Or Fifteen Years That Lifts Worked By
JAN. 1882. 119 ON ECYDRAULIC LIFTS FOR PASSENGERS AND (fOOD8. - BY MB. EDWARD BAYZAND- ELLINGTON, OF LONDOX. It is only within the last ten or fifteen years that Lifts worked by mechanical power have come into general use ; and, excluding docks and railway goods stations, it is still rather the exception than the rule to find power lifts in public buildings, or in warehouses and hotels even of considerable size. The greatly increased value of land in large towns, and the consequent increased height of the buildings erected, render however some kind of mechanical lifting power essential to the comfort and convenience of tho occupiers. Accidents to lifts, especially when worked by mechanical means, have been so frequent, that many hesitate to adopt them on account of the risk involved. But in a rapidly increasing number of cases their use is a neaeasity, and the risk must be taken. It becomes therefore a question of public importance that this risk should be reduced to a minimum. In determining the question as to the best kinds of lift for passengers and for goods, it is necessary to premise that whatever system of lift is proved to be safest and best for passengers should also be adopted, where practicable, for goods. Workmen and others are in the habit of travelling in goods lifts, and a prohibition against this practice is productive of inconvenience, Considerations of expense however will often stand in the way of the adoption of the safest kind of lift for goods alone, especially when the height of lift is great ; and there is in consequence a demand for two standard types, one for passengers and another for goods. -

Electric Lifts and Cranes. 11
Proceedings.1 RAVENSHAW ON ELECTRIC LIFTS AND CRANES. 11 30 March, 1897. JOHN WOLBE BARRY, C.B., F.R.S., President, in the Chair. (Paper No. 3038. ) “ Electric Lifts and Cranes.” By HENRYWILLOCK RAVENSHAW, Assoc. M. Inst. C.E. THE object of this communication is to directattention to the electrical and mechanical problems which present themselves it1 the application of electric motors to working lifts and cranes ; and todescribe the methods bywhich they have been solved in particular cases which have come under the notice of the Author. ELECTRICLIFTS. Thesimple gearing and slowly-moving mechanism necessary wherehydraulic power is available afford many advantages. Withthe electric motor, however, thecurrent varies with the work developed, while with the usual form of hydraulic motor the quantity of water used is as great with a light as with a heavy load. The greater simplicity of the hydraulic lift would therefore probably be an insuficient advantage to justify its in- troduction in places where electricity was already available, and where no hydraulic installation existed. Motor.-The class of electric motor generally employed does not callfor special description. Any good commercial machine can be used, a principal necessity being that it should require little attention.The machinesshould be sparkless under wide varia- tions of load, the brushes self-adjusting for wear, and the direction of rotation reversible. The bearings should also be self-lubricating. The efficiency should be high at all loads ; but a comparatively small machine can be used, as in most cases the maximum load is of short duration, the machine standing idle for at least half the time. -

Discussion. the Transmission of Power to Distances
30 MINUTES OF PROCEEDINGS. Mr. STEPIIENSON,President, said the distribution of power was of considerableimportance inall large undertakings, especially with aview of saving manual labour, which was frequently a source of greater trouble than all themachinery put together. Mr. E. A. COWPERbelieved this was the first instance in which a good arrangement for the distribution of power by water in a town had been described at the Institution. The way in which the work had been carried out appeared tobe excellent. In considering the question of hydraulicmachinery, what Joseph Bramahhad proposed, andwhat he actually did, ought not to be forgotten. In a letterto the late Mr.Robert Mallet, dated November loth, 1802, he distinctly foreshadowed this way of dis- tributing power, andhis letter seemed almost likean original suggestion of the plan which had been described.l Mr. Cowper thoughtnot only would suchuseful applications of hydraulic power bcmuch extended, but that other applications would be made of theprinciple, which was one thatentailed very little friction, even when great force was generated. He had applied a “ hydraulic reservoir,” as early as 1846, to work hydraulic presses for bending,shearing, punching out large plates, and welding iron. All the large end adjustment links for the Kieff bridge, in Russia, were cut out of l-inch plate by a press so worked. But the idea of a ‘Lhydraulic reservoir ” was not new, even then, as it had been used years before in a paper mill for working presses. The hydraulicpress was avaluable tool, and easily managed. There was no difficulty about the leathers, pipes, valves, or any of the appliances, if properly carried out. -

Tm 55-1915-200-10 Technical Manual Operator's Manual
TM 55-1915-200-10 TECHNICAL MANUAL EQUIPMENT DESCRIPTION 1-11 OPERATING INSTRUCTIONS 2-1 PREVENTIVE MAINTENANCE 2-251 OPERATOR'S MANUAL CHECKS AND SERVICES (PMCS) FOR This copy is a reprint which includes current pages from Changes 1 through 6. LOGISTICS SUPPORT VESSEL (LSV) NSN 1915-01-153-8801 Approved for public release. Distribution is unlimited. HEADQUARTERS, DEPARTMENT OF THE ARMY 11 APRIL 1988 TM 55-1915-200-10 C 12 CHANGE HEADQUARTERS DEPARTMENT OF THE ARMY NO. 12 WASHINGTON, D.C., 14 October 1999 Operator’s Manual FOR LOGISTICS SUPPORT VESSEL (LSV) NSN 1915-01-153-8801 DISTRIBUTION STATEMENT A: Approved for public release; distribution is unlimited. TM 55-1915-200-10, 11 April 1988, is changed as follows: 1. Remove and insert pages as indicated below. New or changed text material is indicated by a vertical bar in the margin. An illustration change is indicated by a miniature pointing hand. Remove pages Insert pages i and ii i and ii 1-1 and 1-2 1-1 and 1-2 2-251 through 2-298.14 2-251 through 2-297/(2-298 blank) -- 2028-2 forms 2. Retain this sheet in front of manual for reference purposes. By Order of the Secretary of the ERIC K. SHINSEKI General, United States Army Officia l: Chief of Staff JOEL B. HUDSON Administrative Assistant to the Secretary of the Army DISTRIBUTION: To be distributed in accordance with DA Form 12-25-E, block no. 4207, requirements for TM 55-1915-200-10. TM 55-1915-200-10 C 11 CHANGE HEADQUARTERS DEPARTMENT OF THE ARMY NO. -

Side, Stern and Front Doors
TECHNICAL INFORMATION RoRo Side, stern and front doors Planning the side, stern or front door Our aim is to design a MacGregor door which is efficient, economic and safe, while fully meeting the specific operating require ments. The more information that can be given on these prerequisites, the greater the chance to find the optimum solution. Under the following headings information requirements can found before commencing the project. If these answers are available at the earliest stage possible, work will be saved during the later stages, gaining valuable time by shortening the lead time between initial contact and delivery. Door type and size Loadings Interface between door and ship In order to establish the type and size of In order to dimension the steel structure When you design the hull in the area the door we require information about we need to know the following: of the door, adequate space must be the purpose of the door and drawings of reserved for the door and its associated • the ship’s area of operation (shortsea, the ship. Has the ship double hull, a side equipment. deepsea etc) casing or open web construction? Is the In most cases the door requires a door located in a straight or curved area • the vessel speed space of about 150 mm around the of the shell? • where the door is located (in the front perimeter for the rubber packing. Are there transverse bulkheads, bulkhead, stern, side shell or close to In addition space is needed for both stairways or other items intruding in the the bow) stowing and operation of the door. -

5 Modern Electrical Dock-Equipment, with Special
JAN. 1911. 5 MODERN ELECTRICAL DOCK-EQUIPMENT, WITH SPECIAL REFERENCE TO ELECTRICALLY-OPERATED COAL-HOISTS. - BY MR. WALTER DIXON AND MR. GEORGE H. BASTER, Membeis, GLASGOW. OF - At the Summer Meeting of the Institution of Mechanical Engineers, held at Cardiff in July 1906, three Papers dealing with Coal Shipping and Dock Appliances were read. The general impression in these Papers, and particularly in the discussion thereon, appeared to be that hydraulic power was the only power sufficiently reliable for such appliances, and that Electricity, no matter what other fields it may enter, was certainly not suitable for dock equipments, particularly coaling-cranes and tips. A review of the history of what had already been done with electric power in docks may have justified such an impression, although as far back as 1903, Mr. Walter Pitt, in a Paper before the Institution of Civil Engineers, showed that electric jib-cranes compared very favourably on all points with those operated by hydraulics, quoting some figures obtained by Mr. Baxter, as a result of a comparison between a hydraulic crane and an electric crane at Downloaded from pme.sagepub.com at The University of Melbourne Libraries on June 5, 2016 6 ELECTRICAL DOCK-EQUIPMENT. JAX. 1911. Princes Dock, Glasgow. British Dock Engineers have confined themselves very largely to electric jib-cranes and capstans, which until recently were chiefly modifications of the hydraulic type, very little original design being employed, the electrical plant being simply applied to machines already designed. The authors fully appreciate all the merits of hydraulics, and that this form of power, having been thoroughly developed, is very safe and reliable, and, excepting where frost, moving ground or other natural objections arise, is, perhaps, under favourable conditions, not inferior to electric power. -
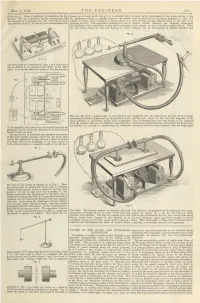
•X* F/ // (©/ I E1 Fv FIC 5 ■Y V End Being Fixed on a Brass Lever P, Figs
May 5, 1882. THE ENGINEER. 319 of the box I. Discs of cardboard K strengthen the dia placed so as to move in the line a & it is attracted when induces certain phenomena to take place, such as in the phragm. Two air channels, L and M, communicate with the diaphragms vibrate in opposite phase to the central case of a piece of iron becoming magnetic, or that of a the two parts of I, and end at L1, M1, where the connect position 1; while when vibrating in similar phase it is wire moving through different parts of the field of an ing tubes can be fixed. H1 has only one communication, the attracted to 3 or 31. This experiment is analogous to one of electric current. Similarly the vibrating diaphragms Dr. Bjerkness ’ with a small piece of iron and bar magnets, cause changes in their immediate neighbourhood. These H the iron being placed on cork and floating in water changes can be investigated as regards direction and B FIG. 10. h ' O E \ lill 1 ■A=: \ /r ~Ez f ij A ■•X* f/ // (©/ i E1 fV FIC 5 ■y v end being fixed on a brass lever P, Figs. 5 and 10, so that it can be shifted to or connected with either of the other 3 ends. It is obvious that a movement of B in the direction i V H E R m. M F1C.S -0 fly! •§ |G> oi Ifc l]rfl I Jj=E== c c C A iSpSi £==55-g? (i I - o ! =■ E UI [ 0 BC |° c jl Q 0 I C c Q ...0 \ ho /////////////////. -

Ac-5101 Engineering Chemistry
AC-5101 ENGINEERING CHEMISTRY L T P Credits:4 3 1 0 UNIT-I REDOX REACTION AND ELECTRO CHEMSITRY: Electrolytic conductance (specific, equivalent and molar conductance), factors affecting conductance, strong and weak electrolytes, Kohlrausch’s law, Effect of dilution on ionic and equivalent conductance, Oxidation, reduction, oxidation number, redox reactions in terms of oxidation number, oxidation reduction as electron transfer process, equivalent weights of oxidizing and reducing agents, Electrochemical cell, Types of electrodes, electrode potential, EMF, cell reactions, EMF of galvanic cell, electrochemical series and its applications, Nernst’s equation, relationship of EMF with equilibrium constant and free energy, primary and secondary batteries, Fuel Cells (acid, alkaline and carbonate). (10 Hrs) CORROSION: Direct, chemical corrosion and mechanism, electrochemical corrosion and mechanism, Galvanic corrosion, concentration cell corrosion, atmospheric corrosion, passivity, pitting corrosion, factors influencing corrosion, prevention of corrosion. (04 Hrs) UNIT-II LUBRICANTS: Classification of lubricants, lubricating oils, semisolid lubricants, solid and synthetic lubricants. Properties of lubricating oils (viscosity, flash and fire points, cloud and pour point, mechanical stability and saponification number). (04 Hrs) PHASE RULE: Introduction, definitions, Gibbs phase rule, phase diagrams, one component systems (water, carbon dioxide, sulfur system), two component systems (Pb-Ag, KI-water, Na2SO4-water system). (05 Hrs) UNIT-III ANALYTICAL -

The North Western Museum of Science and Industry
The North Western Museum of Science and Industry. 1 The North Western Museum of Science and Industry, Some Reminiscences. By Richard L. Hills, M.A., D.I.C., Ph.D., C.I.Mech.E., F.M.A., Dip.Ed. The North Western Museum of Science and Industry. 2 Contents Introduction Chapter 1 A Successful Start at Grosvenor Street. Chapter 2 Problems Facing the Museum. Chapter 3 The Power Hall at Liverpool Road. Chapter 4 The Mill Engine Collection. Chapter 5 The Railway Collection. Chapter 6 The Textile Collection. Chapter 7 The Machine Tool Collection. Chapter 8 Paper Related Exhibits. Chapter 9 Smaller Exhibits. Appendix 1 Lists of some of the exhibits, 1971 – 1977. Appendix 2 Visitor Figures, 1970 – 1983. Appendix 3 Holding of railway, tramroad rails and plates. Appendix 4 Holding of mechanical point and signal lever frames and interlocks. Appendix 5 Catalogue of library and archive collections, circa 1982. Appendix 6 List of attendees at the Royal Visit to the Greater Manchester Museum of Science and Industry, 5 May 1982. Appendix 7 Education Service. Bibliography. The North Western Museum of Science and Industry. 3 Introduction Donald Cardwell, Professor in History of Science and Technology, University of Manchester Institute of Science and Technology, wrote:- “The principal aim of the Museum [would be] to explain the major discoveries and inventions of history of science and technology using wherever possible exhibits made in or linked with the North West, establishing a valuable tool for school and universities… “Thus the Manchester Science Museum should give prominence to textiles, electrical engineering and computers among other things, but pay much less to motorcars, cycles and firearms.