Perineal Raphe with Special Reference to Its Extension to the Anus: a Histological Study Using Human Fetuses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anatomy of the Rectum and Anal Canal
BASIC SCIENCE identify the rectosigmoid junction with confidence at operation. The anatomy of the rectum The rectosigmoid junction usually lies approximately 6 cm below the level of the sacral promontory. Approached from the distal and anal canal end, however, as when performing a rigid or flexible sigmoid- oscopy, the rectosigmoid junction is seen to be 14e18 cm from Vishy Mahadevan the anal verge, and 18 cm is usually taken as the measurement for audit purposes. The rectum in the adult measures 10e14 cm in length. Abstract Diseases of the rectum and anal canal, both benign and malignant, Relationship of the peritoneum to the rectum account for a very large part of colorectal surgical practice in the UK. Unlike the transverse colon and sigmoid colon, the rectum lacks This article emphasizes the surgically-relevant aspects of the anatomy a mesentery (Figure 1). The posterior aspect of the rectum is thus of the rectum and anal canal. entirely free of a peritoneal covering. In this respect the rectum resembles the ascending and descending segments of the colon, Keywords Anal cushions; inferior hypogastric plexus; internal and and all of these segments may be therefore be spoken of as external anal sphincters; lymphatic drainage of rectum and anal canal; retroperitoneal. The precise relationship of the peritoneum to the mesorectum; perineum; rectal blood supply rectum is as follows: the upper third of the rectum is covered by peritoneum on its anterior and lateral surfaces; the middle third of the rectum is covered by peritoneum only on its anterior 1 The rectum is the direct continuation of the sigmoid colon and surface while the lower third of the rectum is below the level of commences in front of the body of the third sacral vertebra. -

THE PHYSIOLOGY and ECOPHYSIOLOGY of EJACULATION Tropical and Subtropical Agroecosystems, Vol
Tropical and Subtropical Agroecosystems E-ISSN: 1870-0462 [email protected] Universidad Autónoma de Yucatán México Lucio, R. A.; Cruz, Y.; Pichardo, A. I.; Fuentes-Morales, M. R.; Fuentes-Farias, A.L.; Molina-Cerón, M. L.; Gutiérrez-Ospina, G. THE PHYSIOLOGY AND ECOPHYSIOLOGY OF EJACULATION Tropical and Subtropical Agroecosystems, vol. 15, núm. 1, 2012, pp. S113-S127 Universidad Autónoma de Yucatán Mérida, Yucatán, México Available in: http://www.redalyc.org/articulo.oa?id=93924484010 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Tropical and Subtropical Agroecosystems, 15 (2012) SUP 1: S113 – S127 REVIEW [REVISIÓN] THE PHYSIOLOGY AND ECOPHYSIOLOGY OF EJACULATION [FISIOLOGÍA Y ECOFISIOLOGÍA DE LA EYACULACIÓN] R. A. Lucio1*, Y. Cruz1, A. I. Pichardo2, M. R. Fuentes-Morales1, A.L. Fuentes-Farias3, M. L. Molina-Cerón2 and G. Gutiérrez-Ospina2 1Centro Tlaxcala de Biología de la Conducta, Universidad Autónoma de Tlaxcala, Tlaxcala-Puebla km 1.5 s/n, Loma Xicotencatl, 90062, Tlaxcala, Tlax., México. 2Depto. Biología Celular y Fisiología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, Ciudad Universitaria, 04510, México, D.F., México. 3Laboratorio de Ecofisiologia Animal, Departamento de Fisiologia, Instituto de Investigaciones sobre los Recursos Naturales, Universidad Michoacana de San Nicolás de Hidalgo, Av. San Juanito Itzicuaro s/n, Colonia Nueva Esperanza 58337, Morelia, Mich., México * Corresponding author ABSTRACT RESUMEN Different studies dealing with ejaculation view this Diferentes estudios enfocados en la eyaculación, process as a part of the male copulatory behavior. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -
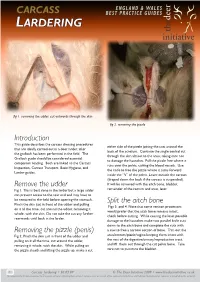
Introduction Remove the Udder Removing the Pizzle (Penis)
fig . removing the udder, cut outwards through the skin fig 2. removing the pizzle Introduction This guide describes the carcass dressing procedures either side of the pizzle joining the cuts around the that are ideally carried out in a deer larder, after back of the scrotum. Continue the single central cut the gralloch has been performed in the field. The through the skin almost to the anus, taking care not Gralloch guide should be considered essential to damage the haunches. Pull the pizzle free where it companion reading. Both are linked to the Carcass runs over the pelvis, cutting the blood vessels. Use Inspection, Carcass Transport, Basic Hygiene, and the knife to free the pizzle where it turns forward Larder guides. inside the “V” of the pelvis. Leave outside the carcass (draped down the back if the carcass is suspended). Remove the udder It will be removed with the aitch bone, bladder, Fig 1. This is best done in the larder but a large udder remainder of the rectum and anus, later. can prevent access to the rear end and may have to be removed in the field before opening the stomach. Split the aitch bone Pinch the skin just in front of the udder and pulling Figs 3. and 4. Note that some venison processors on it all the time, cut around the udder, removing it would prefer that the aitch bone remains intact, whole, with the skin. Do not take the cut any further check before cutting. While causing the least possible rearwards until back in the larder. -

How to Increase Your Enjoyment of Sex
BETTER SEX BETTER SEX BETTER SEX BETTER SEX BETTER SEX OF SEX ENJO YOUR INCREASE HOW TO for women and women for their partners YMENT “Sexual health is a state of physical, emotional, mental and social well-being in relation to sexuality; it is not merely the absence of disease, dysfunction or infirmity. Sexual health requires a positive and respectful approach to sexuality and sexual relationships, as well as the possibility of having pleasurable and safe sexual experiences, free of coercion, discrimination and violence. For sexual health to be attained and maintained, the sexual rights of all persons must be respected, protected and fulfilled.” Definition of sexual health, World Health Organisation SAFER SEX Using condoms for penetrative sex is the best way to protect yourself and your partners from Sexually Transmitted Infections, including HIV. Condoms also offer good protection from unwanted pregnancy. In the text of this booklet, we have chosen not to refer constantly to the use of condoms. Instead, we encourage you to make your own decisions about protecting yourself and others in each instance of sexual activity you undertake. 1 HOW TO INCREASE YOUR ENJOYMENT OF SEX This leaflet provides information on how to help yourself improve your enjoyment of sex. It has three main parts: Suggestions on how to improve sex generally, without doing formal exercises. These apply to both casual and regular partners. Exercises you can do on your own — for women who have difficulty getting turned on or experiencing orgasm, and who may or may not have a regular partner. Exercises you can do with a partner — for women who have difficulty getting turned on, or who have difficulty having an orgasm or enjoying penetrative sex. -
Female Perineum Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Female Perineum Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the student should be able to describe the: ✓ Boundaries of the perineum. ✓ Division of perineum into two triangles. ✓ Boundaries & Contents of anal & urogenital triangles. ✓ Lower part of Anal canal. ✓ Boundaries & contents of Ischiorectal fossa. ✓ Innervation, Blood supply and lymphatic drainage of perineum. Lecture Outline ‰ Introduction: • The trunk is divided into 4 main cavities: thoracic, abdominal, pelvic, and perineal. (see image 1) • The pelvis has an inlet and an outlet. (see image 2) The lowest part of the pelvic outlet is the perineum. • The perineum is separated from the pelvic cavity superiorly by the pelvic floor. • The pelvic floor or pelvic diaphragm is composed of muscle fibers of the levator ani, the coccygeus muscle, and associated connective tissue. (see image 3) We will talk about them more in the next lecture. Image (1) Image (2) Image (3) Note: this image is seen from ABOVE Perineum (In this lecture the boundaries and relations are important) o Perineum is the region of the body below the pelvic diaphragm (The outlet of the pelvis) o It is a diamond shaped area between the thighs. Boundaries: (these are the external or surface boundaries) Anteriorly Laterally Posteriorly Medial surfaces of Intergluteal folds Mons pubis the thighs or cleft Contents: 1. Lower ends of urethra, vagina & anal canal 2. External genitalia 3. Perineal body & Anococcygeal body Extra (we will now talk about these in the next slides) Perineum Extra explanation: The perineal body is an irregular Perineal body fibromuscular mass. -

The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

Penile Circular Fasciocutaneous Flaps for Complex Anterior Urethral Strictures K.J
18 Penile Circular Fasciocutaneous Flaps for Complex Anterior Urethral Strictures K.J. Carney, J.W. McAninch 18.1 Penile Fascial Anatomy – 146 18.2 Flap Anatomy – 148 18.3 Patient Selection – 148 18.4 Preoperative Preparation – 148 18.5 Patient Positioning – 148 18.6 Flap Harvest – 149 18.7 Stricture Exposure – 150 18.8 Anastomosis – 151 18.9 Postoperative Care – 152 References – 152 146 Chapter 18 · Penile Circular Fasciocutaneous Flaps for Complex Anterior Urethral Strictures Surgical reconstruction of complex anterior urethral stric- Buck’s fascia is a well-defined fascial layer that is close- tures, 2.5–6 cm long, frequently requires tissue-transfer ly adherent to the tunica albuginea. Despite this intimate techniques [1–8]. The most successful are full-thickness association, a definite plane of cleavage exists between the free grafts (genital skin, bladder mucosa, or buccal muco- two, permitting separation and mobilization. Buck’s fascia sa) or pedicle-based flaps that carry a skin island. Of acts as the supporting layer, providing the foundation the latter, the penile circular fasciocutaneous flap, first for the circular fasciocutaneous penile flap. Dorsally, the described by McAninch in 1993 [9], produces excel- deep dorsal vein, dorsal arteries, and dorsal nerves lie in a lent cosmetic and functional results [10]. It is ideal for groove just deep to the superficial lamina of Buck’s fascia. reconstruction of the distal (pendulous) urethra, where The circumflex vessels branch from the dorsal vasculature the decreased substance of the corpus spongiosum may and lie just deep to Buck’s fascia over the lateral aspect jeopardize graft viability. -

Critical Androgen-Sensitive Periods of Rat Penis and Clitoris Development Michelle Welsh,* David J
international journal of andrology ISSN 0105-6263 ORIGINAL ARTICLE Critical androgen-sensitive periods of rat penis and clitoris development Michelle Welsh,* David J. MacLeod,* Marion Walker, Lee B. Smith and Richard M. Sharpe MRC Human Reproductive Sciences Unit, Centre for Reproductive Biology, The Queen’s Medical Research Institute, Edinburgh, UK Summary Keywords: Androgen control of penis development/growth is unclear. In rats, androgen androgens, clitoris, flutamide, hypospadias, action in a foetal ‘masculinisation programming window’ (MPW; e15.5–e18.5)’ masculinisation programming window, predetermines penile length and hypospadias occurrence. This has implications micropenis, penis length for humans (e.g. micropenis). Our studies aimed to establish in rats when andro- gen action/administration affects development/growth of the penis and if deficits Correspondence: Richard M. Sharpe, MRC Human Reproductive in MPW androgen action were rescuable postnatally. Thus, pregnant rats were Sciences Unit, Centre for Reproductive treated with flutamide during the MPW ± postnatal testosterone propionate Biology, The Queen’s Medical Research (TP) treatment. To assess penile growth responsiveness, rats were treated with Institute, 47 Little France Crescent, Edinburgh, TP in various time windows (late foetal, neonatal through early puberty, puberty EH16 4TJ, UK. E-mail: [email protected] onset, or combinations thereof). Phallus length, weight, and morphology, hypo- *Contributed equally to the manuscript. spadias and anogenital distance (AGD) were measured in mid-puberty (d25) or Re-use of this article is permitted in adulthood (d90) in males and females, plus serum testosterone in adult males. accordance with the Terms and Conditions MPW flutamide exposure reduced adult penile length and induced hypospadias set out at http://www3.interscience.wiley. -

Bowel Function Anatomy
BOWEL FUNCTION ANATOMY Most of America gives little thought to bowel control. However, bowel control is actually a complex process involving the coordination of many different muscles and nerves. The bowel is considered to be a part of the digestive or gastrointestinal system. It is designed to help the body absorb nutrients and fluids from the foods we eat and drink. After taking out everything the body needs, the bowel then expels the leftover waste. The beginning of the bowel is the small intestine, sometimes referred to as the small bowel. This is where the useful nutrients are absorbed from what you eat. The small bowel delivers the waste to the colon, or large bowel. The colon is a 5-6 foot long muscular tube that delivers stool to the rectum. As the stool moves through the colon, the fluids are removed and absorbed into the body. The consistency of the stool is dependent upon many things, including how long the stool sits in the colon, how much of the water has been absorbed from the waste, and the amount of fiber and fluids in your diet. Stool consistency can vary from hard lumps to mushy to very loose, watery stool. The best and easiest consistency of stool is soft, like toothpaste; this consistency may be attained by adding fiber to your diet. Fiber helps move waste through the colon because it is indigestible by the human body. In other words, fiber adds ‘bulk’ to the stool. It is important to eat a diet high in fiber, however, most Americans lack fiber in their diet. -

Peyronie's Disease
Peyronie’s Disease Blase Prosperi1 and Mang Chen, MD2 1. Georgetown University School of Medicine 2. University of Pittsburgh School of Medicine Introduction Peyronie’s disease is a connective tissue disorder characterized by the formation of fibrous plaques and scar tissue in the erectile tissue of the penis. Patients typically experience symptoms during erections as the scar tissue forces the penis to curve, making sexual intercourse painful and often impossible. Peyronie’s disease occurs most frequently in males aged 40-60 years old, but can also be found in men as young as 18.1 Penile Anatomy and Physiology The penis is a sexual organ that also acts as a conduit for urine. It is composed of three cylinders or chambers: two corpora cavernosa, and one corpus spongiosum. During sexual arousal, the corpora cavernosa fill with blood, creating an erection. The cavernosa run the length of the penis and are attached proximally to the pubic arch. Distally, the cavernosa fuse to the glans—which is the distal aspect of the corpus spongiosum. Situated superiorly to the cavernosa are paired dorsal arteries, paired dorsal nerves, and the superficial and deep vein of the penis. The superficial vein lies superior to the deep fascial layer of the penis (Buck’s fascia); the deep vein, paired arteries, and nerves lie inferior to this layer. The superficial vein drains to the branches of the internal pudendal vein, and the deep vein drains into the prostatic plexus. The corpus spongiosum lies ventral to the two cavernosa and contains the urethra. The spongiosum is anchored at its base to the perineal membrane and then extends to form the ventral body and glans of the penis. -

Refractory Period
اختﻻﻻت عمل جىسی َ آمُزش َمطاَري ٌذف کلی درس : آضىایی با اختﻻﻻت عمل جىسی َ آمُزش مىاسب در ایه مُارد اٌذاف کلی جلسات : ) جٍت ٌر جلسً یک ٌذف ( آضىایی با فازٌای مختلف سیکل پاسخ جىسی آضىایی با عُاملی ماوىذ سه ، دارَ ، بیماریٍا َ ......... تأثیر آوٍا بر مسائل جىسی آضىایی با مسائل جىسی در دَران وُزادی ، وُپایی ، خردسالی ، پیص از مذرسً َ دَران مذرسً آضىایی با مسائل جىسی دردَران بلُغ َ وُجُاوی آضىایی با فعالیتٍای جىسی َتغییرات آن در دَران بارداری َ ضیردٌی آضىآ آضىایی با کٍىسالی َ تأثیر آن بر مسائل جىسی آضىایی با بیماریٍای مختلف َ تأثیر آن ٌا بر مسائل جىسی آضىایی با اختﻻﻻت عمل جىسی َ طریقً مقابلً با آن Anatomy of the male reproductive system Testis Scrotum Penis Seminal vessicles, prostate gland, bulbourethral glands Epididymis, vas deferens, urethra TESTES Primary reproductive organs or gonads Production of sperm Suspended outside the body cavity by scrotum PENIS Deposits sperm in female Erectile tissue (vascular) Erection results from gorging tissue with blood Erection is a parasympathetic spinal reflex to tactile and other stimulation enhanced by sympathetic inhibition Provide the bulk of the semen, a mixture of secretions, sperm and mucous Fructose and prostaglandins from seminal vessicles Alkalinity and clotting enzymes from prostate Lubricant for intercourse from bulbourethral glands Epididymus, vas deferens, urethra: ejaculation Route of exit of sperm Ductus deferns stores sperm During emmission phase of ejaculation sperm are emptied into urethra by sympathetically induced contractions Motor neuron induced contractions