Extactive Distillation in the Separation of Five Close Boiling Alcohol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multicomponent Distillation
01/12/2009 Multicomponent distillation F. Grisafi 1 01/12/2009 Introduction The problem of determining the stage and reflux requitirements for multicomponen t distill a tions ismuch more complex than for binary mixtures. With a multicomponent mixture, fixing one component composition does not uniquely determine theother component compositions and the stage temperature. Also when the feed contains more than two components it is not possible to specify the complete composition of the top and bottom products independently. The separation between the top and bottom products is usually specified by setting limits on two "key components", between which it is desired to make the separation. 2 01/12/2009 Calculation procedure The normal procedure for a typical problem is to solve the MESH (Material balance, Equilibrium, Summation and Heat) balance equations stage-by-stage, from the top and bottom of the column toward the feed point. For such a calculation to be exact, the compositions obtained from both the bottom-up and top-down calculations must mesh at the feed point and mesh the feed composition. The calculated compositions will depend on the compositions assumed for the top and bottom products at the commencement of the calculations. Though it ispossibletomatch the key components, theother components will not match unless the designer was particularly fortunate in choosing the trial top and bottom compositions. 3 01/12/2009 Calculation procedure For a comppyletely rigorous solution the compositions must be adjusted and the calculations repeated until a satisfactory match at the feed point is obtained by iterative trial-and-error calculations. Clearly, the greater the number of components, the more difficult the problem. -

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

SAFETY DATA SHEET Cyclohexane BDH1111
SAFETY DATA SHEET Cyclohexane BDH1111 Version 1.3 Revision Date 03/25/2015 Print Date 05/08/2015 SECTION 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Cyclohexane MSDS Number : 000000011713 Product Use Description : Solvent Manufactured for : VWR International LLC Radnor Corporate Center Building One Suite 200 100 Matsonford Road Radnor PA 19087 For more information call : (Monday-Friday,8.00am-5:00pm) 1-800-932-5000 In case of emergency call : (24 hours/day, 7 days/week) 1-800-424-9300(USA Only) For Transportation Emergencies: 1-800-424-9300 (CHEMTREC - Domestic) 1-613-996-6666 (CANUTEC - Canada) SECTION 2. HAZARDS IDENTIFICATION Emergency Overview Form : liquid, clear Color : colourless Odor : mild sweet Page 1 / 15 SAFETY DATA SHEET Cyclohexane BDH1111 Version 1.3 Revision Date 03/25/2015 Print Date 05/08/2015 Classification of the substance or mixture Classification of the substance : Flammable liquids, Category 2 or mixture Skin irritation, Category 2 Specific target organ toxicity - single exposure, Category 3, Central nervous system Aspiration hazard, Category 1 GHS Label elements, including precautionary statements Symbol(s) : Signal word : Danger Hazard statements : Highly flammable liquid and vapour. May be fatal if swallowed and enters airways. Causes skin irritation. May cause drowsiness and dizziness. Precautionary statements : Prevention : Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Keep container tightly closed. Ground/bond container and receiving equipment. Use explosion-proof electrical/ ventilating/ lighting/ equipment. Use only non-sparking tools. Take precautionary measures against static discharge. Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray. Wash skin thoroughly after handling. Use only outdoors or in a well-ventilated area. -
Chapter 13.Pptx
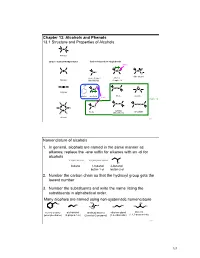
Chapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic O C C C N C N C X O nitro alkane X= F, Cl, Br, I amines Alkenes Alkyl Halide Chapter 23 OH C C H O C O C C O C C Alkynes phenol alcohols ethers epoxide acidic Chapter 14 H H H C S C C C C S S C C S C C H C C sulfides thiols disulfide H H (thioethers) Arenes 253 Nomenclature of alcohols 1. In general, alcohols are named in the same manner as alkanes; replace the -ane suffix for alkanes with an -ol for alcohols CH3CH2CH2CH3 CH3CH2CH2CH2OH OH butane 1-butanol 2-butanol butan-1-ol butan-2-ol 2. Number the carbon chain so that the hydroxyl group gets the lowest number 3. Number the substituents and write the name listing the substituents in alphabetical order. Many alcohols are named using non-systematic nomenclature H C OH 3 OH OH C OH OH HO OH H3C HO H3C benzyl alcohol allyl alcohol tert-butyl alcohol ethylene glycol glycerol (phenylmethanol) (2-propen-1-ol) (2-methyl-2-propanol) (1,2-ethanediol) (1,2,3-propanetriol) 254 127 Alcohols are classified according to the H R C OH C OH H H degree of substitution of the carbon bearing H H 1° carbon the -OH group methanol primary alcohol primary (1°) : one alkyl substituent R R C OH C OH R R secondary (2°) : two alkyl substituents H R 2° carbon 3° carbon tertiary (3°) : three alkyl substituents secondary alcohol tertiary alcohol Physical properties of alcohols – the C-OH bond of alcohols has a significant dipole moment. -

A Case Study on Separation of IPA-Water Mixture by Extractive Distillation Using Aspen Plus
International Journal of Advanced Technology and Engineering Exploration, Vol 3(24) ISSN (Print): 2394-5443 ISSN (Online): 2394-7454 Research Article http://dx.doi.org/10.19101/IJATEE.2016.324004 A case study on separation of IPA-water mixture by extractive distillation using aspen plus Sarita Kalla, Sushant Upadhyaya*, Kailash Singh, Rajeev Kumar Dohare and Madhu Agarwal Department of Chemical Engineering, Malaviya National Institute of Technology, Jaipur, India ©2016 ACCENTS Abstract Extractive distillation is one of the popular methods being leveraged to separate isopropyl alcohol (IPA) from water present in waste stream in semi-conductor industries. In this context, the paper aims to carry out simulation study for separation of isopropyl alcohol-water azeotrope mixture using ethylene glycol as entrainer. In particular, temperature, pressure and concentration profile has been studied. Aspen plus® (version 8.8) has been used as simulation tool and optimum number of stages for the simulation turns out to be 42. The results show that top of the column contains IPA of 99.974 mol % purity and bottom product contains water-ethylene glycol mixture. Moreover, sensitivity analysis for different parameters and analysis of residue curve for ternary system have been also performed. Keywords IPA-water, Extractive distillation, Simulation, Entrainer. 1. Introduction 2. Process simulation Isopropyl alcohol is generally used as the cleaning 2.1 Thermodynamic model agent and solvent in chemical industries. Because of Thermodynamic model is used to describe phase its cleaning property it is also known as rubbing equilibria properties of the system. For the design of alcohol. IPA is soluble in water and it forms chemical separation operations the phase equilibrium azeotrope with water at temperature 80.3-80.4 0C. -

A New Approach to Prepare Polyethylene Glycol Allyl Glycidyl Ether
E3S Web of Conferences 267, 02004 (2021) https://doi.org/10.1051/e3sconf/202126702004 ICESCE 2021 A new approach to prepare Polyethylene Glycol Allyl Glycidyl Ether Huizhen Wang1*, Ruiyang Xie1, Mingjun Chen1*, Weihao Deng1, Kaixin Zhang2, Jiaqin Liu1 1School of Science, Xihua University, Chengdu 610039, China; 2Chengdu Jingyiqiang Environmental Protection Technology Co., Ltd. Abstract. The polyethylene glycol allyl glycidyl ether (PGAGE) is an important intermediate for preparing silicone softener that can be synthesized from allyl alcohol polyoxyethylene ether and epichlorohydrin (ECH). The performance parameters including the concentration of ECH, initial boron trifluoride diethyl etherate (BFEE) as well as CaCl2 quality were investigated respectively. The optimum process parameters which can get high capping and low by-product rate are as follows: the ECH concentration is 2.0 M, the initial BFEE concentration is 1.65mM, and the CaCl2 dosage is 1.65g/L. Under these conditions, the maximal yield can be improved to 91.36%, the percent of capping rate is higher than 98.16%, the residual concentration of F- is only 0.63 mg/L. concentrated basic solution, in which the total yield was between 90%~91% by Matsuoka et al. [10] also use the 1 Introduction two-step reaction to synthesize AGE based on the reaction Polyethylene glycol allyl glycidyl ether (PGAGE) and the of allyl alcohol with ECH using BFEE as the catalyst. allyl polyoxyethylene ether (APEG), tethering with both Their results demonstrated that the yield reaches 82% alkene and epoxy groups, are widely used as fabric under the following condition: n (ECH) : n (allyl alcohol): finishing agent [1-2] , reactive diluent [3] , cross-linking (catalysis) = 1: (1~3) : (0.01~0.002). -

Safety Data Sheet: Cyclohexane
Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH), amended by 2015/830/EU Cyclohexane ROTISOLV® ≥99,9 %, UV/IR-Grade article number: CP81 date of compilation: 2018-05-03 Version: 2.0 en Revision: 2020-10-06 Replaces version of: 2018-05-03 Version: (1) SECTION 1: Identification of the substance/mixture and of the company/ undertaking 1.1 Product identifier Identification of the substance Cyclohexane ROTISOLV® ≥99,9 %, UV/IR-Grade Article number CP81 Registration number (REACH) 01-2119463273-41-xxxx Index No 601-017-00-1 EC number 203-806-2 CAS number 110-82-7 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: laboratory chemical laboratory and analytical use 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone: +49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data : Department Health, Safety and Environment sheet: e-mail (competent person): [email protected] 1.4 Emergency telephone number Name Street Postal code/ Telephone Website city National Poisons Inform- Beaumont Road Dublin 9 01 809 2166 https://www.poisons.ie/ ation Centre Beaumont Hospital SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 (CLP) Classification acc. to GHS Section Hazard class Hazard class and cat- Hazard egory state- ment 2.6 flammable liquid (Flam. -

Liquid-Liquid Extraction
OCTOBER 2020 www.processingmagazine.com A BASIC PRIMER ON LIQUID-LIQUID EXTRACTION IMPROVING RELIABILITY IN CHEMICAL PROCESSING WITH PREVENTIVE MAINTENANCE DRIVING PACKAGING SUSTAINABILITY IN THE TIME OF COVID-19 Detecting & Preventing Pressure Gauge AUTOMATIC RECIRCULATION VALVES Failures Schroedahl www.circor.com/schroedahl page 48 LOW-PROFILE, HIGH-CAPACITY SCREENER Kason Corporation www.kason.com page 16 chemical processing A basic primer on liquid-liquid extraction An introduction to LLE and agitated LLE columns | By Don Glatz and Brendan Cross, Koch Modular hemical engineers are often faced with The basics of liquid-liquid extraction the task to design challenging separation While distillation drives the separation of chemicals C processes for product recovery or puri- based upon dif erences in relative volatility, LLE is a fication. This article looks at the basics separation technology that exploits the dif erences in of one powerful and yet overlooked separation tech- the relative solubilities of compounds in two immis- nique: liquid-liquid extraction. h ere are other unit cible liquids. Typically, one liquid is aqueous, and the operations used to separate compounds, such as other liquid is an organic compound. distillation, which is taught extensively in chemical Used in multiple industries including chemical, engineering curriculums. If a separation is feasible by pharmaceutical, petrochemical, biobased chemicals distillation and is economical, there is no reason to and l avor and fragrances, this approach takes careful consider liquid-liquid extraction (LLE). However, dis- process design by experienced chemical engineers and tillation may not be a feasible solution for a number scientists. In many cases, LLE is the best choice as a of reasons, such as: separation technology and well worth searching for a • If it requires a complex process sequence (several quali ed team to assist in its development and design. -

Example: Multicomponent Distillation with Shortcut Methods Consider A
2 Example: multicomponent distillation with shortcut methods Consider a single distillation column as shown in Fig. 6-1. Short-cut methods have been developed that allow you to see the splits that can be achieved at different pressures, with different reflux ratios, and with a different number of stages. It is often useful to do a short-cut distillation calculation first, before doing the more rigorous plate- to-plate calculations, because the preliminary calculations give you an idea of what is reasonable. The methods are based on the relative volatility of the chemicals, and require you to identify two components that will be essentially separated (to the extent you specify). If you line up all the components in the order of their boiling points, and draw a line between two of them, the more volatile component is called the light key and the less volatile component is called the heavy key. B1 Figure 6-1. Distillation Column For this example, you choose the first column in the process shown in Fig. 5-4. In Aspen Plus you use the module DSTWU for the shortcut method; you also use RK-Soave as the physical property method, because it is a good one for hydrocarbons. The feed is 100 lb. moles/hr of propane, 300 lb. moles/hr of i-butane, and the other chemicals as listed in Table 5-1 or Table 6-6, at 138 psia and 75 ºF. The column operates at 138 psia with a reflux ratio of 10 (a wild1 guess initially, confirmed because the column worked). -

Enhanced Butanol Production by Free and Immobilized Clostridium Sp
Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells Using Butyric Acid as Co-Substrate Laili Gholizadeh This thesis comprises 30 ECTS credits and is a compulsory part in the Master of Science with a Major in Chemical Engineering – Applied Biotechnology 120 ECTS credits No. 10/2009 Title: Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells using Butyric Acid as Co-Substrate. Author: Laili Gholizadeh Baroghi (e-mail: [email protected]) Master Thesis Subject Category: Biotechnology (Bioprocess Engineering – Biofuels) University College of Borås School of Engineering SE-501 90 BORÅS Telephone: (+46) 033 435 4640 Examiner: Prof. Mohammad Taherzadeh Supervisor and Thesis Advisor: Prof. Shang–Tian Yang Supervisor Address: OSU–Ohio State University 125 Koffolt Laboratories 140 West 19th Ave. Columbus, OH 43210–1185, USA Client: Ohio State University (OSU), Chemical & Biomolecular Engineering Department Prof. Shang–Tian Yang Columbus, Ohio; USA. Date: 08–12–2009 Keywords: Bio-butanol y Acetone–Butanol–Ethanol (ABE) y ABE- fermentation y Butyric acid y Clostridium y C. acetobutylicum ATCC 824 y C. beijerinckii ATCC 55025 y C. beijerinckii BA 101 y C. beijerinckii NCIMB 8052 y Fibrous-bed Bioreactor (FBB) y Batch y Suspended cell culture y Immobilized cell system. DEDICATION I would like to dedicate this M.Sc. Thesis to my beloved Family for all their love and encouragement and for always been supportive of my choices. “I am among those who think that science has great beauty. A scientist in his laboratory is not only a technician: he is also a child placed before natural phenomena, which impress him like a fairy tale.” − Marie Curie ABSTRACT Butanol production by four different Clostridium sp. -

Cyclohexane Oxidation Continues to Be a Challenge Ulf Schuchardt A,∗, Dilson Cardoso B, Ricardo Sercheli C, Ricardo Pereira A, Rosenira S
Applied Catalysis A: General 211 (2001) 1–17 Review Cyclohexane oxidation continues to be a challenge Ulf Schuchardt a,∗, Dilson Cardoso b, Ricardo Sercheli c, Ricardo Pereira a, Rosenira S. da Cruz d, Mário C. Guerreiro e, Dalmo Mandelli f , Estevam V. Spinacé g, Emerson L. Pires a a Instituto de Qu´ımica, Universidade Estadual de Campinas, P.O. Box 6154, 13083-970 Campinas, SP, Brazil b Depto de Eng. Qu´ımica, Universidade Federal de São Carlos, 13565-905 São Carlos, SP, Brazil c College of Chemistry, University of California, Berkeley, CA 94720, USA d Depto Ciências Exatas e Tecnológicas, Universidade Estadual de Santa Cruz, 45650-000 Ilhéus, BA, Brazil e Universidade Federal de Lavras, Lavras, MG, Brazil f Instituto de Ciências Biológicas e Qu´ımicas, PUC-Campinas, 13020-904 Campinas, SP, Brazil g Sup. Caracterização Qu´ımica, IPEN, 05508-900 São Paulo, SP, Brazil Received 3 October 2000; received in revised form 21 December 2000; accepted 28 December 2000 Abstract Many efforts have been made to develop new catalysts to oxidize cyclohexane under mild conditions. Herein, we review the most interesting systems for this process with different oxidants such as hydrogen peroxide, tert-butyl hydroperoxide and molecular oxygen. Using H2O2, Na-GeX has been shown to be a most stable and active catalyst. Mesoporous TS-1 and Ti-MCM-41 are also stable, but the use of other metals such as Cr, V, Fe and Mo leads to leaching of the metal. Homogeneous systems based on binuclear manganese(IV) complexes have also been shown to be interesting. When t-BuOOH is used, the active systems are those phthalocyanines based on Ru, Co and Cu and polyoxometalates of dinuclear ruthenium and palladium.