Plant Resource Journal 2020 Final.Pmd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Short Listed Candidates for the Post
.*,ffix ryryffi-ffiffiWffir@fuffir. rySW{Jrue,€ f,rc, "*,*$,S* S&s#ery W€ryff$,,,SffWryf ryAeW Notice for the Short Listed Candidates applying in the post of "Trainee Assistantrr Details fot Exam: a. 246lestha,2076: Collection of Entrance Card (For Surrounding Candidates). b. 25nJestha, 2076: Collection of Entrance Card 8:00AM to 9:45AM in Exam Center (For Others). (Please carry Original Citizenship Certificate and l passport size photo). Written Test (Exam) : Date : 25'hJesth, 2076 Saurday (8ftJune, 2019). Time : 10:00 AM to 11:30 AM tVenue : Oxford Higher Secondary School, Sukhkhanagar, Butwal, Rupandehi. Paper Weightage : 100 Marks Composition Subjective Questions : 02 questions @ 10 marks = 20 marks Objective Questions : 40 questions @ 02 marks = B0 marks Contents 1,. General Banking Information - 10 objective questions @ 2 marks = 20 marks 2. Basic Principles & Concept of Accounting - 10 objective questions @2marks = 20 marks 3. Quantitative Aptitude 10 objective questions @ 2 marks = 20 marks 4. General ISowledge - 10 objective questions @ 2 marks = 20 marks (2 Subjective questions shall be from the fields as mentioned above). NOTE: ,/ I(ndly visit Bank's website for result and interview notice. For Futther Information, please visit : Ffuman Resource Department Shine Resunga Development Bank Limited, Central Office, Maitri Path, But'ural. enblrz- *rrc-tr fffir tarmrnfrur *ffi ffi{" ggffrus gevrcgemmrup w mes'g:iwme ffiAru${ frffi. S4tq t:e w r4r4.q klc S.No. Name of Applicant Address 7 Aakriti Neupane Sainamaina-03, M u reiva RuoanriEh] -

District Public Health Office, Rupandehi of the Year FY 2070/071
Government of Nepal Ministry of Health and Population Phone: 071-520260 Department of Health Services 071-520142 071-525331 Western Region Health Directorate Fax: 071-520840 District Public Health Office Email: [email protected] Rupandehi Acknowledgement It is my great pleasure to publish the Annual Report of District Public Health Office, Rupandehi of the year FY 2070/071. This report is the summary of performance of each program with trend analysis of last 3 fiscal years' services provided by the health facilities (SHPs, HPs, PHCs, and Hospitals), PHCs/ORCs, EPI Clinics, I/NGOs and Nursing homes and private and teaching hospitals. This report is prepared with untiring efforts and co-operation of many institution and individuals. I would like to extend my sincere gratitude to Mr. Bal Krishna Bhusal, Director of Western Regional Health Directorate (WRHD), Pokhara for his valuable direction and guidance provided during district level monitoring visits in different time periods. My sincere thanks go to Mr. Rishi Ram Sigdel, Statistical Officer of WRHD and Mr. Mukti Khanal, Section Chief from Department of Health Services, Mgmt Division, HMIS section for their technical assistance on time and again and in particular during annual review meeting. Additionally, I take this opportunity to express appreciation to all DPHO Supervisors including Admin and Finance staff, Health Workers, Local bodies, Volunteers (FCHVs), Health Facility Management Committees, District level partners working for the quality assurance and enhancement of health services. I would like to thank Mr. Prayash Khanal, Executive Director of Unity for Sustainable Community Development and SUAAHARA program (Rupandehi) team, Mr. Dinesh Poudyal, Team Leader of Namuna Integrated Development Council and Mr. -
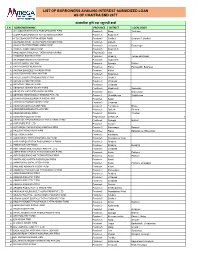
List of Borrowers Availing Interest Subsidized Loan As of Chaitra End 2077
LIST OF BORROWERS AVAILING INTEREST SUBSIDIZED LOAN AS OF CHAITRA END 2077 व्यवशायिक कृ यि तथा पशुपꅍछी कर्ाा S.N BORROWER NAME PROVINCE DISTRICT LOCAL BODY 1 A CLASS KRISHI TATHA PASHUPANCHHI FIRM Province5 Dang Tulshipur 2 A ONE PASHUPANCHHI TATHA MATSYA FARM Province5 Rupandehi 3 A TO Z BANGUR TATHA KRISHI FARM Province3 Sindhuli Golanjor-7, Sindhuli 4 AADHUNIK KRISI TATHA PASHUPANKSHI FIRM Province6 Dailekh 5 AAGYA MULTIPURPOSE AGRO FARM Province3 Chitawan Ratnanagar 6 AAMA CHHORI AGRO FARM Province5 Rupandehi 7 AAMCHOWK PHALPHUL TATHA KRISHI FARM PROVINCE1 Ilam 0 8 AAMDADA BAKHRA FARM Province3 Dhading Jwalamukhi,Maidi 9 AARAMBHA EKIKRIT KRISHI FIRM Province5 Rupandehi 10 AARATI GAIPALAN FIRM Province4 Syangja Bhirkot 11 AARYA BHAISIPALAN FIRM Province2 Parsa Parsagadhi, Bagnawa 12 AASTHA BAHUDESIYA KRISHI FIRM Province7 Kailali 13 AASUTOSH PASHUPALAN FIRM Province5 Rupandehi 14 AAYUS JUNAR UTPADAN KRISHI FIRM Province3 Sindhuli 15 AAYUSHA POULTRY FIRM Province3 Chitawan 16 AAYUSHA POULTRY FIRM Province3 Chitawan 17 ABHISHEK AKIKRIT KRISHI FARM Province5 Rupandehi Sudodhan 18 ABHISHEK HATCHERY MACHHA FIRM Province2 Bara Bishrampur 19 ABHIYAN KRISHI BIKASH KENDRA PVT LTD Province1 Okhaldhunga Siddicharan 20 ACHARYA BAHUUDASHYA KRISHI FIRM Province4 Kaski 21 ACHARYA PASHUPANCHHI FARM Province3 Chitawan 22 ADHIKARI AGRICULTURE FIRM province1 Terhathum Bhanu 23 ADHIKARI BAKHARA PALAN FIRM Province6 Dailekh Bhairabi 24 ADHIKARI KRISHI FIRM Province3 Dolakha Charikot 25 ADHIKARI POULTRY FIRM PROVINCE4 SYANGJA 0 26 ADHIKHOLA PASHUPANCHHI -

Annual Progress Report FY 2070/71
A joint programme of the Government of Nepal in collaboration with the Government of Finland, Switzerland, and the UK Multi Stakeholder Forestry Programme (MSFP) Western Terai Lot III (Nawalparasi, Rupandehi and Kapilvastu) Annual Progress Progress Report F.Y. 070/71 (Reporting Period 16 July 2013 - 16 July 2014) Submitted by Resource Identification and Management Society (RIMS), Nepal P.O. Box 2464, Kathmandu Tel. No: 5537613 Email: [email protected] Website: www.rimsnepal.org Annual Progress Report FY 2070/71 Table of Contents Acronyms ................................................................................................................................................ 3 Chapter 1 Basic Information ................................................................................................................ 4 1.1 Strategic Review and Outlook ................................................................................................. 4 1.2 Way Forward ........................................................................................................................... 5 1.3 Introduction .................................................................................................................................. 6 1.3.1 Snapshot of Nepal .................................................................................................................. 6 1.3.2 Introduction of Implementing Agency: RIMS Nepal .............................................................. 6 1.3.3 Implementation of Multi Stakeholder -

Wetland Flora of Rupandehi District, Nepal
2019J. Pl. Res. Vol. 17, No. 1, pp 58-68, 2019 Journal of Plant Resources Vol.17, No. 1 Wetland Flora of Rupandehi District, Nepal Kalpana Sharma (Dhakal)*, Dammar Singh Saud and Nirmala Joshi Department of Plant Resources, Thapathali, Kathmandu, Nepal *Email: [email protected] Abstract The present study was carried out to document the wetland flora of three wetlands of Rupandehi district during the year of 2016-2018. Macrophytes plant specimens were collected up to 5 m around the wetland. Altogether 115 species belonging to 45 families were recorded. Out of these, 33 species were alien which include 12 invasive species that seems wetland flora were in threats. Keywords: Alien Species, Conservation, Invasive Plants, Local Use, Macrophytes Introduction In Nepal, about 10% of ethnic communities depended on wetlands resources for the subsistence. National Wetland Policy (2003) defines wetlands as The Nepalese wetlands consist of many threatened natural or artificially created areas, such as swamp, and endangered flora and fauna and provide marsh, riverine flood plain, lake, water storage area excellent ecological habitats for internationally and agricultural land containing water from underground important winter migratory birds, aquatic fauna and water resources or atmospheric precipitation that other wildlife (IUCN, 2004). One species of may be permanent or temporary, static or flowing, protected plants under the Forest Regulation 1994 and freshwater or saline. Chaudhary (1998) such as Dalbergia latifolia as well as wild cultivar explained wetland dependent flora as the plants that of rice such as Oryza rufipogon, Oryza nivara, Oryza flourish well in wetland habitats such as marshes, officinalis are known recorded from Terai wetlands swamps, floodland, in rivers or river banks. -

Survey of Cultivated Vegetable Crops in Rupandehi District, Nepal
Himalayan Biodiversity 5: 24-31, 2017Prithvi, Vol 6: , July 2013 ISSN :2382-5200 SURVEY OF CULTIVATED VEGETABLE CROPS IN RUPANDEHI DISTRICT, NEPAL Chandra Bahadur Thapa Butwal Multiple Campus, Butwal [email protected] ABSTRACT Vegetable is very nutritious food and is considered to be protective food since it contains high amount of vitamins and minerals and also possesses medicinal value. In the present study, documentation of farmer’s knowledge on cultivated vegetable crops was carried out in Rupandehi district during the year 2016. The objective of this paper is to identify, enumerate and to know the status of vegetable crops in this district. It was carried out by conducting semi-structured interview with the vegetable growing farmers, local people, members of Community Based Organizations with the help of standard questionnaire, checklist, Focus Group Discussion (FGD) and key informant interview. Altogether 50 plant species have been found to be cultivated in commercial scale as vegetable crops in Rupandehi district. Out of 50 plant spp.; 2 families, 4 genera and 5 spp. were monocots; and 9 families, 30 genera and 45 spp. were dicots. It is also found that fruit (55%) is the widely used part of plant as vegetable. Other parts like leaf (21%), inflorescence (4%), root (8%), tuber (2%), corm (6%), and bulb (4%) are also used as vegetable. Most of the vegetable growing farmers (91%) are economically benefited by the cultivation and selling of vegetable than other crops due to easily available seeds, fertilizers and pesticides in market; good facility of irrigation and accessible market in the study area. -

The Convention on Biological Diversity
NEPAL'S SIXTH NATIONAL REPORT TO THE CONVENTION ON BIOLOGICAL DIVERSITY Government of Nepal Ministry of Forests and Environment (MoFE) Singha Durbar, Kathmandu, Nepal December 2018 NEPAL'S SIXTH NATIONAL REPORT TO THE CONVENTION ON BIOLOGICAL DIVERSITY Government of Nepal Ministry of Forests and Environment (MoFE) Singha Durbar, Kathmandu, Nepal December 2018 NEPAL'S SIXTH NATIONAL REPORT TO THE CONVENTION ON BIOLOGICAL DIVERSITY PREPARED & PUBLISHED BY: Government of Nepal Ministry of Forests and Environment Singha Durbar, Kathmandu, Nepal Telephone: +977-1-4211567 Toll Free Number: 16600101000 Fax : 977-1-4211868 Email : [email protected] THE NATIONAL REPORT PREPARATION COORDINATION TEAM 1. Yajna Nath Dahal, Environment and Biodiversity Division Chief, MoFE : Coordinator 2. Gopal Prasad Bhattarai, DDG, DNPWC : Member 3. Buddi Sagar Paudel, DDG Forest Research and Training Centre : Member 4. Rajendra KC, DDG Department of Forest and Soil Conservation : Member 5. Mohan Joshi, DDG, Department of Plant Resource : Member 6. Bidhya Pandey, Ministry of Agriculture and Livestock Development : Member 7. Representative of Central Department of Environmental Science, TU : Member 8. Representative of Department of Environment, KU : Member 9. Chiranjivi Prasad Pokharel, NTNC : Member 10. Shailendra Giri, ZSL Nepal : Member 11. Representative of WWF Nepal : Member 12. Amit Poudel, IUCN Nepal : Member 13. Ishana Thapa, BCN : Member 14. Vijay Prasad Kesari UNDP Nepal : Member 15. Ramchandra Khatiwada, Biodiversity Section Chief, MoFE : Member Secretary and Contact person CONSULTANT TEAM Prof. Dr. Ram Prasad Chaudhary Dr. Shalu Adhikari The Ministry of Forests and Environment encourages the usage of this material for educational and non- profit purpose, with appreciate credit to the publisher. -

I Health Education, Prefeasibility and Feasibility Studies, Design and Construction Supervision of the Water Supply Schemes
I .... I (IRQ His Majesty's Government of Nepal I Ministry of Housing and Physical Planning DEPARTMENT OF WATER SUPPLY AND SEWERAGE 1 and The Government of Finland I Ministry for Foreign Affairs FINNISH INTERNATIONAL DEVELOPMENT AGENCY I (FINNIDA) I I RURAL WATER SUPPLY AND SANITATION PROJECT I LUMBINI ZONE I ANNUAL REPORT 1991 I I I " IMSTAHI ILLUSTRATION I tram unicat C4KHI I I I I Butwal, February 1992 I Consultant: PLANCENTER LTD Helsinki I I -A/pi.teg2-/0 I I reports\anl'rep.92 I List of abbreviations I CHV = Community Health Volunteer I CWSS = Community Water Supply and Sanitation DEO = District Educational Officer I DPHO = District Public Health Officer DWE = District Water Engineer I DWSO = District Water Supply Office DWSS = Department of Water Supply and Sewerage FIM = Finnish Mark I FINNIDA = Finnish International Development Agency HESP = Health Education and Sanitation Programme of RWSSP I HMG/N = His Majesty's Government of Nepal HP = Health Post I HRD = Human Resources Development IOM = Institute of Medicine I MHPP = Ministry of Housing and Physical Planning MOEC = Ministry of Education MOH = Ministry of Health I NER = Nepalese Rupee NPC = National Planning Commission I PHC = Primary Health Care PIU = Project Implementation Unit I RAP = Rapid Assessment Procedure RD = Regional Director I RWSSP = Rural Water Supply and Sanitation Project UC = User's Committee UMN = United Mission to Nepal I UNICEF = United Nations Children Fund USD = United States Dollar I VHW = Village Health Worker WHO = World Health Organization I WSST = Water Supply and Sanitation Technician LIBRARY, INTERNATIONAL REFERENCE CENTRE FOR GOMiV.UNiTY WATER SUPPLV I AND 3AN4TAT1ON1 (ItfC) P.O. -
![Xf]D Axfb'/ &Fs"/ Cfs[Lt Kgyl ऋृषिराम प](https://docslib.b-cdn.net/cover/1276/xf-d-axfb-fs-cfs-lt-kgyl-7451276.webp)
Xf]D Axfb'/ &Fs"/ Cfs[Lt Kgyl ऋृषिराम प
खुला प्रलतयोलगता配मक परीक्षाको वीकृ त नामावली वबज्ञापन नं. : २०७७/७८/३३ (लुम्륍बनी प्रदेश) तह : ३ पदः कलनष्ठ सहायक (गो쥍ड टेटर) रोल नं. उ륍मेदवारको नाम उ륍मेदवारको नाम ललंग सम्륍मललत हुन चाहेको समूह थायी म्ि쥍ला थायी न. पा. / गा.वव.स बािेको नाम बाबुको नाम 1 AACHAL SINGH THAKUR आँचल l;+x &fs"/ Female ख쥍ु ला, महिला Banke Sub- Metropolitan Nepalgunj eujtL l;+x &fs"/ xf]d axfb'/ &fs"/ 2 AAKRITI PANTHI cfs[lt kGyL Female ख쥍ु ला, महिला Gulmi Thanapati ऋृषिराम पन्थी बाबुराम पन्थी 3 AAKRITI GIRI cfs[tL lu/L Female ख쥍ु ला, महिला Rolpa Sunil Smiriti vu" lu/L zf]ef/fd lu/L 4 AARATI KHANAL cf/lt vgfn Female ख쥍ु ला, महिला Tehrathum Solma lglwgfy vgfn led k|;fb vgfn 5 AARTI PARIYAR आरती पररयार Female ख쥍ु ला, महिला Banke Nepalgunj भोटे ष िंह पररयार षबष्ण ु पररयार 6 AASHA BASEL cfiff a;]n Female ख쥍ु ला, महिला Pyuthan Belbas VDC l/vL/fd h};L k"gf/fd h};L 7 AASHA GHIMIRE cfzf l#ld/] Female ख쥍ु ला, महिला Arghakhanchi patauti lxdnfn l#ld/] sdn k|;fb l#ld/] 8 AASTHA SHRESTHA cf:yf >]i& Female ख쥍ु ला, महिला Rupandehi Butwal dVvg nfn >]i& lji)f" nfn >]i& 9 AAWSAHAYAK MANI PANDEY अवशायक मषण पाण्डoे Male ख쥍ु ला Rupandehi Rohini षशतल प्र ाद पाण्डे षदनकर मषण पाण्डे 10 AAYUSHMA HAMAL K.C. -

Sample Design: Sample Frame: Target Population
Sample design: Two stage sample design was adopted following assumption to get reliable estimate and achieve the objectives. The main objectives are to find actual vital and migration registration numbers among total events occurred in the society. In addition to know the trend of vital registration system in last three years. Sample Frame: Sample frame of CRVS survey was constructed from National Population census 2011. This frame has aggregated ward wise information about House Number, Household Number, and male, female and total population. However the provided frame was based on 2011 administrative boundaries which were reconstructed into new existing administrative boundaries having 217 municipalities. Here basically wards are our enumeration area (EA). Target population: There are often several target populations in household surveys. In CRVS survey, the following events occurred in last three years were our target population. (1) Children less than three years, (2) Households having death, (3) Married couple, (4) Divorce persons, (5) In & out migrated household and (6) Adoption of Children. Sample size depends also on the target population so that the calculation of the sample size must therefore take into consideration each of the target populations. The resulting sample size was considerably larger than that needed for a target group comprised of all persons or all households. Domains: Major significant factor that has a large impact on the sample size is the number of domains. Domains are generally defined as the analytical sub-groups for which equally reliable data are wanted. Domains of study were 15 ecological development areas and Kathmandu valley altogether 16 domains. Poilot survey: Before determining the sample size, some of required parameter should be known and updated. -

Social Structure, Livelihoods and the Management of Common Pool Resources in Nepal
ANNEX A Social Structure, Livelihoods and the Management of Common Pool Resources in Nepal Final Report NRSP Project R7975 September 2003 Overseas Development Group (ODG) University of East Anglia, Norwich NR4 7TJ Natural and Organisational Resources Management Services (NORMS) P.O. Box 3671, Maitidevi, Kathmandu ANNEX A Project Overview: This research investigated the links between new systems of management of common pool resources and existing social and political relations around natural resource use. It was based on the premise that new systems of resource management are embedded within existing social and political relations, and that an understanding of these is essential for the successful design and implementation of new policies. The research focused on two districts, Rupandehi and Nawalparasi, in the Terai region of Nepal and sought to map information on the livelihoods of different social groups with access to forests and forest products. This information is used to examine the implications of proposed and actual changes being implemented in forest resource management, especially for more vulnerable groups, and to suggest ways in which participative management approaches that are designed to improve resource access of poorer groups might need to be modified to enhance the livelihood security of these groups. Our research highlights the importance of understanding the broader institutional setting in which `forest user groups' operate and that merely adjusting the membership criteria of the FUGs, for example, cannot ensure that poor women and men have access and control of the CPRs that they need to sustain their livelihoods. Keywords: Social structure and social and processes, livelihood security, poverty, natural resource management, common pool resources, Nepal Project location: Five fieldwork locations in two districts in the Terai of southern Nepal (selected from Nawalparasi and Rupandehi). -

District Public Health Office, Rupandehi of the Year FY 2069/070
Government of Nepal Ministry of Health and Population Phone: 071-520260 Department of Health Services 071-520142 071-525331 Western Region Health Directorate Fax: 071-520840 Email: [email protected] District Public Health Office Rupandehi Acknowledgement It is my great pleasure to publish the Annual Report of District Public Health Office, Rupandehi of the year FY 2069/070. This report is the summary of performance of each program with trend analysis of last 3 fiscal years services provided by the health facilities (SHPs, HPs, PHCs, and Hospitals), PHC/ORC, EPI Clinic, NGO and Nursing homes and private and teaching hospitals. This report is prepared with untiring efforts and co-operation of many institution and individuals. I would like to extend my sincere gratitude to Dr.Ashok Kumar Chaurasia, Director of Western Regional Health Directorate (WRHD), Pokhara for his valuable direction and guidance provided during district level monitoring visits in different time periods. My sincere thanks go to Mr. Rishi Ram Sigdel, Statistical Officer and Mr Rajendra Paudel of WRHD; and Mr. Mukti Khanal,Section Chief and Mr. Surya Khadga from Department of Health Services, Mgmt Division, HMIS section for their technical assistance on time and again and in particular during annual review meeting. Additionally, I take this opportunity to express appreciation to all DPHO Supervisors including Admin and Finance staff, Health Workers, Local bodies, Volunteers (FCHVs), Health Facility Management Committees, District level partners working for the quality assurance and enhancement of health services. I would like to thank Mr. Deepak Tiwari, Project Manager of CARE Nepal and team, Mr.