Austrapen Ampicillin (As Sodium) Powder for Injection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNASYN® (Ampicillin Sodium/Sulbactam Sodium)
NDA 50-608/S-029 Page 3 UNASYN® (ampicillin sodium/sulbactam sodium) PHARMACY BULK PACKAGE NOT FOR DIRECT INFUSION To reduce the development of drug-resistant bacteria and maintain the effectiveness of UNASYN® and other antibacterial drugs, UNASYN should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION UNASYN is an injectable antibacterial combination consisting of the semisynthetic antibiotic ampicillin sodium and the beta-lactamase inhibitor sulbactam sodium for intravenous and intramuscular administration. Ampicillin sodium is derived from the penicillin nucleus, 6-aminopenicillanic acid. Chemically, it is monosodium (2S, 5R, 6R)-6-[(R)-2-amino-2-phenylacetamido]- 3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate and has a molecular weight of 371.39. Its chemical formula is C16H18N3NaO4S. The structural formula is: COONa O CH3 N CH O 3 NH S NH2 Sulbactam sodium is a derivative of the basic penicillin nucleus. Chemically, sulbactam sodium is sodium penicillinate sulfone; sodium (2S, 5R)-3,3-dimethyl-7-oxo-4-thia 1-azabicyclo [3.2.0] heptane-2-carboxylate 4,4-dioxide. Its chemical formula is C8H10NNaO5S with a molecular weight of 255.22. The structural formula is: NDA 50-608/S-029 Page 4 COONa CH3 O N CH3 S O O UNASYN, ampicillin sodium/sulbactam sodium parenteral combination, is available as a white to off-white dry powder for reconstitution. UNASYN dry powder is freely soluble in aqueous diluents to yield pale yellow to yellow solutions containing ampicillin sodium and sulbactam sodium equivalent to 250 mg ampicillin per mL and 125 mg sulbactam per mL. -

Estrogen Metabolism in the Human Estrogen Metabolism in the Human
ENE PURRE: ESTROGEN METABOLISM IN THE HUMAN ESTROGEN METABOLISM IN THE HUMAN A THESIS BY ENE PURRE SUBMITTED TO THE FACULTY OF GRADUATE STUDIES AND RESEARCH IN PARTIAL FULFILMENT FOR THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE McGILL UNIVERSITY MONTREAL, CANADA. DEPARTMENT OF EXPERIMENTAL MEDICINE AUGUST 1965 TABLE OF CONTENTS Page No. I INTRODUCTION A. Early history and isolation of the e strogens 2 B. Metabolism of the estrogens 4 1. Biogenesis 4 2. Metabolism 6 3. Degradation 14 4. Conjugation 15 c. Altered states of estrogen metabolism 18 Purpose of the Investigation II MATERIALS AND METHODS 25 Materials I Radioactive compounds 25 II Non-radioactive compounds 25 Ill Enzymes 26 IV Reagents 26 Methods I Purification of radioactive steroids 30 II Preparation of non-radioactive standards 31 III Spectophotometric and f1uorimetric readings 31 IV Counting methods 32 V Enzyme assays 33 VI Injections 33 VII Collection of urine 34 VIII Hydrolysis and extraction of urines 34 IX Assay of estrone, estradiol-17 f.:' and estriol 36 X Attempts at separa ting ring 011( keto1s 3 7 XI Assay of ring D..: ketols in the urine 39 XII Recrysta1lization studies 40 ---------------···---- TABLE OF CONTENTS (cont.) Page No._ Ill RESULTS Section I - Studies with control subjects and those with myocardial infarction 41 Section II- A. Recovery of 16~-hydroxyestrone and 16-ketoestradiol-17~ from toluene/ propylene glycol system 50 B. Pre 1 imi nary study on urinary ring De>< ketols 51 c. Urinary ring D<K ke to 1 s in nonnal males 53 D. Studies of the excretion and metabolism of eight estrogen metabolites from urine 59 IV DISCUSSION 71 SUMMARY 87 BIBLIOGRAPHY 89 • LIST OF FIGURES Figure No. -

AUGMENTIN® (Amoxicillin/Clavulanate Potassium) Tablets
NDA 50-564/S-051 Page 3 AG:AL16 PRESCRIBING INFORMATION AUGMENTIN® (amoxicillin/clavulanate potassium) Tablets To reduce the development of drug-resistant bacteria and maintain the effectiveness of AUGMENTIN (amoxicillin/clavulanate potassium) and other antibacterial drugs, AUGMENTIN should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION AUGMENTIN is an oral antibacterial combination consisting of the semisynthetic antibiotic amoxicillin and the β-lactamase inhibitor, clavulanate potassium (the potassium salt of clavulanic acid). Amoxicillin is an analog of ampicillin, derived from the basic penicillin nucleus, 6-aminopenicillanic acid. The amoxicillin molecular formula is C16H19N3O5S•3H2O, and the molecular weight is 419.46. Chemically, amoxicillin is (2S,5R,6R)-6-[(R)-(-)-2-Amino-2-(p- hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate and may be represented structurally as: Clavulanic acid is produced by the fermentation of Streptomyces clavuligerus. It is a β-lactam structurally related to the penicillins and possesses the ability to inactivate a wide variety of β-lactamases by blocking the active sites of these enzymes. Clavulanic acid is particularly active against the clinically important plasmid-mediated β-lactamases frequently responsible for transferred drug resistance to penicillins and cephalosporins. The clavulanate potassium molecular formula is C8H8KNO5, and the molecular weight is 237.25. Chemically, clavulanate potassium is potassium (Z)- (2R,5R)-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]-heptane-2-carboxylate, and may be represented structurally as: Inactive Ingredients: Colloidal silicon dioxide, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium starch glycolate, and titanium dioxide. -

The Endocrine Function of the Placenta: Interactions Between Trophic Peptides and Hormones
Placenlal Function and Fetal Nutrition, edited by Frederick C. Battaglia, Nestle Nutrition Workshop Series. Vol. 39. Nestec Ltd.. Vevey/Lippincott-Raven Publishers. Philadelphia. © 1997. The Endocrine Function of the Placenta: Interactions Between Trophic Peptides and Hormones Lise Cedard U. 361 INSERM, Maternite Baudelocque, 121 Boulevard de Port-Royal Paris, France The placenta is classically considered to be a source of hormones that play an important role in the establishment and maintenance of pregnancy. Because of its hemochorial structure, the human placenta produces hormones and easily secretes them into the maternal circulation. They include steroid hormones, such as estrogens and progesterone, and protein hormones, the most important of which are the chori- onic gonadotrophins (hCG), discovered in 1927. During the period from 1960 to 1980, the concept of a fetoplacental unit (1) with a complementary role for the fetus in steroid production offered a new approach to steroid metabolism, and since then new proteins and neuropeptides have been discovered (2,3). However, the lack of innervation of placental tissue highlights the importance of understanding neuroen- docrine regulation of placental physiology. Sophisticated culture methods enabling the study of biologic phenomena occurring during transformation of cytotrophoblast cells into a syncytiotrophoblast (4) have been combined with electron microscopy and histochemistry. Possibilities offered by molecular biology, including in situ hybridization and molecular cloning studies, -

Generic Formulary - 2015 S
GENERIC FORMULARY - 2015 S. N. Composition Strength Form VMS_code NEW VMS 1. Anaesthetics 1 Atropine 0.6 mg / ml Inj G01019 2 Benzocaine 20% Gel G01026 3 Bupivacaine 25mg/10ml Inj G01010 4 Bupivacaine 50mg/10ml Inj G01011 5 Bupivacaine in Dextrose 5% +7.5% Inj G01012 6 Desflurane 250 ml Inhalation G01027 7 Dexmedetomidine 100mg/ml Inj G01028 8 Diazepam 5 mg/ml Inj G01020 9 Doxapram 4 mg/ml Inj G01032 10 Ethyl Chloride 1% Spray G01013 11 Glycopyrroolate 0.2 mg/ml Inj G01029 12 Halothane Inhalation Inhalation G01002 13 Isoflurane Inhalation Inhalation G01003 14 Ketamine 50mg/ml Inj G01005 15 Ketamine 10 mg / ml Inj G01004 16 Lignocaine and Adrenaline 1% + 1:200,000 Inj G01017 17 Lignocaine and Adrenaline 2% + 1:200,000 Inj G01018 18 Lignocaine and Dextrose 5%+7.5% Inj G01016 19 Lignocaine 2% Gel G01014 20 Lignocaine 2,5% Gel G01033 21 Lignocaine 1% INJ G01034 22 Lignocaine 2% INJ G01015 23 Lignocaine 5% GEL G01035 24 Midazolam Injection 1 mg / ml Inj G01021 25 Midazolam Injection 5mg/ml Inj G01022 26 Nitrous Oxide Inhalation Inhalation G01006 27 Oxygen Inhalation Inhalation G01007 28 Propofol Injection 10 mg / ml Inj G01025 29 Sevoflurane 250MG Inhalation G01024 30 Thiopentone Injection I.P 0.5G 0.5G Inj G01008 31 Thiopentone Injection I.P 0.5G 1 G Inj G01009 32 Xylocaine spray 10% 10% Spray G01030 2. A n a l g e s i c s , A n t i p y r e t i c s , N o n s t e ro i d a l Anti-inflammatory Medicines, Medicines used to treat 33 AceclofenacRheumatoid 100mg Disorders. -

Common Formulary of Generic Drugs for Medical Store Organisation, CGHS, Dr
Common Formulary Of Generic Drugs For Medical Store Organisation, CGHS, Dr. R.M.L Hospital, Safdarjung Hospital And Lady Harding Medical College & Kalawati Saran Children Hospital-2011 With VMS Nos (Vocabulary of Medical Stores) Common Formulary Of Generic Drugs For Medical Store Organisation, CGHS, Dr. R.M.L Hospital, Safdarjung Hospital And Lady Harding Medical College & Kalawati Saran Children Hospital With Effect From 23-05-2011 SlNO VMS No Nomenclature Type of Strength Medicine 1. Anaesthetics 1.1 General Anaesthetics and Oxygen 1 G01002 Halothane Inhalation 250ml (Halothane with Inhalation Inhalation vaporizer) 2 G01003 Isoflurane Inhalation Inhalation Inhalation 3 G01004 Ketamine Hydrochloride 10 mg / ml Injection 10 mg / ml 10ml 4 G01005 Ketamine Hydrochloride 10 mg / ml Injection 50mg/ml 10ml 5 G01006 Nitrous Oxide Inhalation Inhalation 6 G01007 Oxygen Inhalation Inhalation Inhalation 7 G01008 Thiopentone Sodium Injection 0.5 g powder 8 G01009 Thiopentone Sodium Injection 1g powder 9 G01024 Sevoflurane Inhalation 250ml Bott Inhalation Inhalation 10 G01025 Propofol Injection 1% 11 G01027 Desflurane 250 ml Inhalation 250 ml 1.2 Local Anaesthetics 12 G01010 Bupivacaine Hydrochloride Injection 0.25% 13 G01011 Bupivacaine Hydrochloride Injection 0.50% 14 G01012 Bupivacaine in Dextrose Injection 0.5% +7.5% 15 G01013 Ethyl Chloride Spray 1% 16 G01014 Lignocaine Hcl Gel 2-5% 17 G01015 Lignocaine Hcl Injection 2% 18 G01016 Lignocaine Hydrochloride and Dextrose Injection 5% +7.5% 19 G01017 Lignocaine Hydrochloride and Adrenaline Injection -

New Zealand Data Sheet 1
New Zealand Data Sheet 1. PRODUCT NAME Ibiamox® 250mg Powder for injection Ibiamox® 500mg Powder for injection Ibiamox® 1000mg Powder for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION The amoxicillin is present as the sodium salt in Ibiamox (1059mg of amoxicillin sodium is equivalent to approximately 1000mg of amoxicillin). Ibiamox 250mg Powder for injection contains 250mg amoxicillin. Ibiamox 500mg Powder for injection contains 500mg amoxicillin. Ibiamox 1000mg Powder for injection contains 1000mg amoxicillin. 3. PHARMACEUTICAL FORM Ibiamox vials: white to cream powder packed in clear glass vials for reconstitution. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications Amoxicillin should be used in accordance with local official antibiotic-prescribing guidelines and local susceptibility data. Amoxicillin is indicated for treatment of infections in adults and children at the following sites, when caused by sensitive organisms: • upper respiratory tract including ear, nose and throat infections, e.g. tonsillitis, sinusitis, otitis media • lower respiratory tract, e.g. acute exacerbations of chronic bronchitis, lobar and bronchopneumonia • gastrointestinal tract, e.g. typhoid fever • genito-urinary tract, e.g. cystitis, urethritis, pyelonephritis, bacteriuria in pregnancy, gonorrhoea, septic abortion, puerperal sepsis • other infections including Borreliosis (Borrelia burgdorferi) (Lyme Disease) • prophylaxis of endocarditis: amoxicillin may be used for the prevention of bacteraemia associated with the development of endocarditis (see table in section 4.2 Dose and method of administration) • Skin and soft tissue infections (SSSIs). Susceptibility to amoxicillin will vary with geography and time and local susceptibility data should be consulted where available and microbiological sampling and susceptibility testing performed where necessary (see section 5.1 Pharmacodynamic properties). 1 | Page 4.2. -
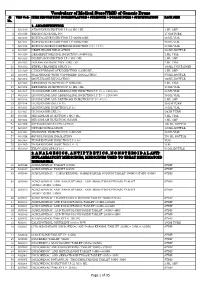
Vocabulary of Medical Store(VMS) of Generic Drugs Sl VMS Code ITEM DESCRIPTION (NOMENCLATURE + STRENGTH + DOSAGE FORM + SPECIFICATION) PACK SIZE No
Vocabulary of Medical Store(VMS) of Generic Drugs Sl VMS Code ITEM DESCRIPTION (NOMENCLATURE + STRENGTH + DOSAGE FORM + SPECIFICATION) PACK SIZE No. 1. ANAESTHETICS 1 G01019 ATROPINE INJECTION I.P 0.6 MG / ML 1 ML AMP 2 G01026 BENZOCAINE GEL 20% 15 GM TUBE 3 G01010 BUPIVACAINE INJECTION I.P 25MG/10ML 20 ML VIAL 4 G01011 BUPIVACAINE INJECTION I.P 50MG/10ML 20 ML VIAL 5 G01012 BUPIVACAINE IN DEXTROSE INJECTION 0.5% +7.5% 20 ML VIAL 6 G01027 DESFLURANE INHALATION 250ML BOTTLE 7 G01028 DEXMEDETOMIDINE INJECTION 100MG/ML 2 ML VIAL 8 G01020 DIAZEPAM INJECTION I.P 5 MG / ML 2 ML AMP 9 G01032 DOXAPRAM INJECTION 4 MG / ML 2 ML VIAL 10 G01013 ETHYL CHLORIDE SPRAY 1% 100ML CONTAINER 11 G01029 GLYCOPYRROOLATE INJECTION 0.2 MG/ML 1 ML AMP 12 G01002 HALOTHANE (WITH VAPORIZER) INHALATION 250ML BOTTLE 13 G01003 ISOFLURANE INHALATION 100ML BOTTLE 14 G01005 KETAMINE INJECTION I.P 50MG/ML 2 ML VIAL 15 G01004 KETAMINE INJECTION I.P 10 MG / ML 10 ML VIAL 16 G01017 LIGNOCAINE AND ADRENALINE INJECTION I.P 1% + 1:200,000 30 ML VIAL 17 G01018 LIGNOCAINE AND ADRENALINE INJECTION I.P 2% + 1:200,000 20 ML VIAL 18 G01016 LIGNOCAINE AND DEXTROSE INJECTION I.P 5% +7.5% 2 ML AMP 19 G01014 LIGNOCAINE GEL I.P 2% 30 GM TUBE 20 G01015 LIGNOCAINE INJECTION I.P 2% 30 ML VIAL 21 G01035 LIGNOCAINE GEL 5% 20GM TUBE 22 G01021 MIDAZOLAM INJECTION 1 MG / ML 5 ML VIAL 23 G01022 MIDAZOLAM INJECTION 5MG/ML 1 ML AMP 24 G01006 NITROUS OXIDE INHALATION 100 ML BOTTLE 25 G01007 OXYGEN INHALATION 100ML BOTTLE 26 G01025 PROPOFOL INJECTION I.P 10MG/ML 20 ML VIAL 27 G01024 SEVOFLURANE INHALATION 250 ML BOTTLE 28 G01008 THIOPENTONE INJECTION I.P 0.5G VIAL 29 G01009 THIOPENTONE INJECTION I.P 1G VIAL 30 G01030 XYLOCAINE SPRAY 10% 50 ML BOTTLE 2. -

Unconjugated Estradiol, Estriol and Total Estriol in Maternal Peripheral
Endocrinol. Japon. 1983, 30 (2), 155-162 Unconjugated Estradiol, Estriol and Total Estriol in Maternal Peripheral Vein, Cord Vein, and Cord Artery Serum at Delivery in Pregnancies with Intrauterine Growth Retardation. CHIKAYUKI, TAYAMA, SHUNZO ICHIMARU, MASAHARU ITO, MICHIO NAKAYANA, MASAO MAEYAMA AND ISAO MIYAKAWA Department of Obstetrics and Gynecology, Kumamoto University Medical School, Kumamoto 860 and *Department of Obstetricts and Gynecology, Miyazaki Medical College, Miyazaki Abstract The levels of unconjugated estradiol(E2), estriol (E3) and total (conjugated plus unconjugated) E3 in maternal vein serum during labor, cord vein serum, and cord artery serum were measured in normal singleton and twin pregnanceis with appropriate for dates babies (AFD) and with light for dates babies (LFD). The mean level of total E3 in the maternal vein serum in singleton pregnancy was significantlylower in the LFD group than in the AFD group, but no differenceswere seen in the mean levels of unconjugated E2 or E3 between the groups. The concentration of uncon- jugated E2 in the maternal vein serum was significantlyhigher in the twin group with a large placenta than in the singleton group with a small placenta, while the concentration of total E3 in the case of twin pregnancy with LFD was lower than that in singleton pregnancy with AFD but not significantly. No difference in the concentration of total E3 was observed between the cord vein serum and cord artery serum. The present data suggest that the total E3 level in maternal vein serum may be used in evaluating fetal states such as intrauterinegrowth retardation. In pregnancy complicated by intrauterine conjugated E2 and E3 into the maternal and growth retardationwith or without symptoms fetal circulation and to determine the total of maternal disease,such as toxemia of pre- (conjugated plus unconjugated) E3 in the gnancy or hypertension,not only the urinary maternal and fetal blood. -

Thesis.Pdf (432.0Kb)
Some hormonal factors in the etiology of endometrial cancer Elisabete Weiderpass Stockholm 1999 Department of Medical Epidemiology, Karolinska Institutet, Stockholm, Sweden Some hormonal factors in the etiology of endometrial cancer Elisabete Weiderpass Stockholm 1999 2 To all women who took part in these studies 3 ABSTRACT The main purpose of this dissertation was to study the impact of some hormone-related factors in the etiology of endometrial neoplasms, i.e. hormone replacement therapy, use of oral contraceptives, serum levels of 20 different organochlorine substances, and polymorphisms in the estrogen receptor α (ER) gene. We conducted two population-based case-control studies among post-menopausal women. In the first one, 789 women with a reported diagnosis of primary endometrial cancer and 3368 age-frequency-matched control women were enrolled from all over Sweden. These women answered a questionnaire on use of oral contraceptives and hormone replacement therapy, among other questions. We used unconditional logistic regression to calculate odds ratios (OR) as estimates of relative risks. We found a duration-dependent increase in the relative risk of endometrial cancer both among women who used orally administered estriol, 1-2 mg/day (multivariate OR following 5 or more years of use: 3.0; 95% confidence interval [CI] 2.0-4.4) and medium-potency estrogens without addition of progestins (multivariate odds ratio following 5 or more years of use: 6.7; 95% CI 4.3-10.5), compared to women who never used these substances. Following combined estrogen-progestin use, the association was considerably weaker (multivariate odds ratio following 5 or more years of use: 1.6; 95% CI 1.1-2.4) than for estrogens without progestins, and the increased relative risk was confined to women using cyclic regimens (i.e. -

Department of Health & Human Services Nda 50-757/S
Public Health Service DEPARTMENT OF HEALTH & HUMAN SERVICES Food and Drug Administration Rockville, MD 20857 NDA 50-757/S-011 PRIOR APPROVAL SUPPLEMENT TAP Pharmaceutical Products Inc. Attention: John R. Lieberman, Ph.D Regulatory Advisor 675 North Field Drive Lake Forest, IL 60045 Dear Dr. Lieberman: Please refer to your supplemental new drug application dated January 31, 2005, received February 1, 2005, submitted under section 505(b)(1) of the Federal Food, Drug and Cosmetic Act for PREVPAC® (lansoprazole/amoxicillin/clarithromycin). This supplemental new drug application provides for the following revisions to the package insert (additions are double underlined and deletions are strikethrough): 1. The TRIMOX® (amoxicillin, USP) subsection of the DESCRIPTION section was revised to read: Amoxicillin, USP, (2S,5R,6R)-6-[( R)-(-)-2-Amino-2-(p-hydroxyphenyl) acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate is a semi-synthetic penicillin antibiotic, an analogue of ampicillin, with a broad spectrum of bactericidal activity against many gram –positive and gram-negative microorganisms. Chemically it is (2S,5R,6R)-6-[( R)-(-)-2-Amino- 2-(p-hydroxyphenyl) acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylic acid trihydrate. Amoxicllin capsules are intended for oral administration. 2. The Amoxicillin: subsection of the CLINICAL PHARMACOLOGY section was revised to read: Most of the amoxicillin is excreted unchanged in the urine; its excretion can be delayed by concurrent administration of probenicid. Amoxicillin is not highly protein bound. In blood serum, amoxicillin is approximately 20% protein bound as compared to 60% for penicillin G. -

Study Protocol Drug Substance Ceftazidime-Avibactam Study Code D4280C00015 Edition Number 2 Date 07 March 2017 Approximately 70 Study Centres Are Planned
.9/L"H(A"L .-4*.LDF KL09E:92L Qe2ne"V`J'+n ')PX-$-9'T/Z!J:Z ^f)kn L),n Z *6Z7LIneE",Pn Z [,n ZC!+Z Z @4 +'@ ( ,@ +.) 4@)8'5!+51@ 6 :@ .,51.''@52 '@6.@; '9 5@ 4 5=@ 5.'7 ' 6=@/ ) .& +6!4@ +@" =@.@5 > *@ +@ ; 5 )@<-@ #;,@ #,@.) + 5 .,@< 5@)51.+! ?.(@ .)0 2@< 5@ 32./+)@ +@ (1,@2.)@ ).+64@6.@'44@5 +@ = 24@ .@ @<$6@ .)0( 6@ ,6 .)!, '@ +5!.+4@% %4@ <97A9=L V`P'I&' n bD),P[;?<,ni,*,In PPD AE="5"L"B">%L5L )!# B(E"L >";?"B"6DE+I"L PPD \,n \f*ln-).Pn PP n D n n PP n,Z4,P?!I)Wn PPD n n + ' *+($"+ (+ (6(AE)H+ '5$"BL*5.G "L(7LD&*AL >"H*A"L;>9E991L %+9L %+9#L&+ 9.L3"53"7EL9L E"L + +%+ Z Z'@J': 'BZ Z C!+Z Z Z 3(6*AE=D(H"L D"L 9#L3*4*AE>E+J"L 92L!3+8+CD@E+J"L %+9#L.9/L 46$+9L '6$"L % '6$"L + 3+4,CD>D+J"L + % % % Y+/HZ4.;-!5ZKQ%VZC?L?!?5Z+HZ '';ZHR3'!MZM?ZZA''DZC'T/'UZ!!?C$-;*ZM?Z L<$C$Z@E?!'%SC'HZ ,'Z !5/>-!5ZHMR$WZ@C?M?!?5Z -HZAR6-!5WZ F'*/HM'C'$Z;$ZJ+'Z G'HR8MHZC!Z &0H#7?H'$Z;%?CZ@S51H+($Z!!?C$-;*Z M?Z INC2';'!Z5?6Z?6/!WZ?;Z-?'M+-!HZ;$Z/;Z Z!?:A5/;!'ZU/O,Z AC'T/5-;*Z 5UHZ;$ZC(*R5L1?=HZ 090177e18e51c7a8\0.1\Draft\Versioned On:09-May-2018 14:11 (GMT) PROTOCOL SYNOPSIS A single blind, randomised, multi-centre, active controlled, trial to evaluate safety, tolerability, pharmacokinetics and efficacy of ceftazidime and avibactam when given in combination with metronidazole, compared with meropenem, in children from 3 months to less than 18 years of age with complicated intra-abdominal infections (cIAIs) International Co-ordinating Investigator PPD , MD PPD United States Study site(s) and number of patients planned A sufficient number of patients are to be randomised 3:1 for 80 patients to complete at least 72 hours (3 full days, ie, 9 doses) of study treatment (ie, evaluable patients; at least 60 patients in the ceftazidime and avibactam [CAZ-AVI] plus metronidazole group and at least 20 patients in the meropenem group).