Technique for Intrascleral Intraocular Lens Fixation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Yamane Technique Modification for Haptic Exposure After Glued Intrascleral Haptic Fixation
American Journal of Ophthalmology Case Reports 14 (2019) 101–104 Contents lists available at ScienceDirect American Journal of Ophthalmology Case Reports journal homepage: www.elsevier.com/locate/ajoc Case report Novel yamane technique modification for haptic exposure after glued intrascleral haptic fixation T ∗ Rachel A. Gelman, Sumit Garg Gavin Herbert Eye Institute, University of California, Irvine, 92697, United States ARTICLE INFO ABSTRACT Keywords: The field of intraocular lens fixation in the setting of inadequate capsular support is a dynamic one as surgical Yamane technique approaches are constantly evolving. There has been a paradigm shift towards the use of sutureless methods of Haptic exposure scleral fixation to avoid suture-related complications. In the latest described style of scleral fixation, IOLs can be Intraocular lens (IOL) secured without suture or “glue”, and rather with the creation of a flange on each haptic that allows for firm intrascleral fixation. We describe a modification of the flange technique to refixate patients with glued IOLs who developed haptic extrusion and required surgical intervention. 1. Introduction 48% of eyes with a sutured IOL. The risk of postoperative glaucoma was also found to be lower, occurring 16% of the time with glued IOLs 1.1. Sutureless techniques for scleral fixation versus 40% with sutured IOLs.5 Furthermore, the use of glue over su- ture precludes problems such as suture breakage and exposure, which In 2007, Agarwal et al. described the use of fibrin glue for fixation of in a retrospective study by McAllister et al. occurred at a rate of 6.1% a posterior chamber IOL in the setting of deficient capsular support in a and 11% respectively with the use of 10–0 polypropylene.2 Degradation technique referred to as “glued IOL fixation”. -

Posterior Chamber Scleral Fixation of Intraocular Lenses in Post
DOI: 10.7860/JCDR/2017/20989.9533 Original Article Posterior Chamber Scleral Fixation of Intraocular Lenses in Post- Ophthalmology Section Vitrectomised Aphakic Eyes FRANCIS KWASI OBENG1, VIPAN KUMAR VIG2, PREETAM SINGH3, RAJBIR SINGH4, BODHRAJ DHAWAN5, NIKHIL SAHAJPAL6 ABSTRACT Materials and Methods: Records of all patients who had Introduction: The best method of aphakia correction is in the undergone secondary PCSFIOL implantation with sutures bag implantation of Posterior Chamber Intraocular Lens (PCIOL). after combined PPV and lensectomy from 2010 to 2014 were When this ideal procedure is not possible due to lack of integrity reviewed retrospectively for visual outcomes and complications. of posterior capsule or zonules, the other alternatives are broadly Patients’ demographic data, indication for PPV, best corrected categorized into two: extraocular and intraocular. Whereas, the preoperative and postoperative visual acuities, complications former includes contact lenses and aphakic glasses, the latter of surgery, and indications of PCSFIOL and length of follow up ones are further divided into anterior and posterior chamber were collected and analyzed. methods. Anterior Chamber Intraocular Lenses (ACIOL) can be Results: A total of 148 eyes of 148 patients (127 males and with or without iris claw. At the posterior chamber, fixation of the 21 females) were identified. Mean age at surgery was 32.5+8 lenses can be with glue or sutures. years (range 2.5-73 years) with a mean follow up 23+14 months When there is combined Pars Plana Vitrectomy (PPV) and (range 3-114 months). A total of 95.27%, 2.70% and 2.02% lensectomy or if the indication of PPV is dropped nucleus or of patients had improvement, maintenance and worsening of intraocular lens, a modality of aphakia correction should be their final postoperative visual acuities respectively. -

DENSE, DISLOCATED, BRUNESCENT CATARACT Approaching Cataract Surgery in a Monocular Patient
CATARACT SURGERY CASE FILES s DENSE, DISLOCATED, BRUNESCENT CATARACT Approaching cataract surgery in a monocular patient. BY ASHVIN AGARWAL, MS; MARJAN FARID, MD; P. DEE G. STEPHENSON, MD, FACS; AND AUDREY R. TALLEY ROSTOV, MD CASE PRESENTATION An 84-year-old Russian woman presents for a cataract surgery evaluation. The patient has a history of a failed graft, retinal detachment, and glaucoma in her right eye. Visual acuity is light perception in her right eye and count- ing fingers in her left. A slit-lamp examination of the patient’s left eye finds a dense cataract, an inferiorly dislocated lens, loose zonules, and phacodonesis (Figure). The cornea is clear, and endothelial cell density is normal. B-scan ultrasonography shows no retinal detachment. The IOP measures 15 mm Hg. Examination reveals no other significant findings. How would you proceed? What form of anesthesia would you use? What would your preferences be in terms of surgical technique and IOL? —Case prepared by Audrey R. Talley Rostov, MD Figure. Examination of the patient’s left eye shows a dense cataract and loose zonules. I would begin by making a conjuncti- were well tucked into the Scharioth val peritomy at the 3 and 9 clock hours tunnels, I would seal them with glue. I and two partial-thickness scleral flaps would end the case with pupilloplasty located 180º apart. I would also create to optimize the outcome. the SICS tunnel but leave it unopened. ASHVIN AGARWAL, MS After performing continuous curvilinear capsulorhexis, in order to ensure that Because the patient is almost blind the zonules were manipulated as little as and her right eye has undergone mul- possible, I would pull the lens into the tiple surgeries, I would first attempt to anterior chamber. -

Glued Capsular Hook: Technique for Fibrin Glue-Assisted Sutureless
TECHNIQUE Glued capsular hook: Technique for fibrin glue–assisted sutureless transscleral fixation of the capsular bag in subluxated cataracts and intraocular lenses Soosan Jacob, MS, FRCS, DNB, Amar Agarwal, MS, FRCS, FRCOphth, Athiya Agarwal, MD, DO, Ashvin Agarwal, MS, Smita Narasimhan, MB BS, Dhivya Ashok Kumar, MD We describe a technique that uses a capsular hook to obtain sutureless fibrin glue–assisted trans- scleral fixation of the capsular bag. The hook passes through a sclerotomy created under a scleral flap and engages the capsulorhexis rim, providing scleral fixation intraoperatively and postoper- atively. A standard capsular tension ring expands the capsular fornix. The haptic of the hook is tucked into a scleral tunnel for postoperative fixation. The scleral flap is closed with fibrin glue. The glued capsular hook is used for subluxated cataracts and IOLs. It anchors the capsular bag to the sclera, providing vertical and horizontal stability, and stabilizes the bag intraoperatively and postoperatively. The technique was used in 7 patients, who were followed for more than 4 months. Financial Disclosure: Dr. Jacob has a patent pending for the glued capsular hook models. No other author has a financial or proprietary interest in any material or method mentioned. J Cataract Refract Surg 2014; 40:1958–1965 Q 2014 ASCRS and ESCRS Online Video The management of subluxated cataracts and intraoc- possibility of long-term suture-related complications. ular lenses (IOLs) depends on the degree of zonulo- We describe a technique that uses a glued capsular dialysis.1,2 For subluxations up to 3 to 4 clock hours, hook (Figure 1) to achieve sutureless fibrin glue–assis- a capsular tension ring (CTR) is often sufficient, but ted transscleral fixation of the capsular bag. -
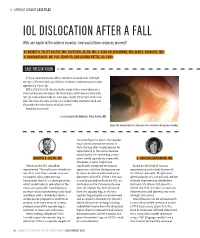
Iol Dislocation After a Fall a After Dislocation Iol Cataract & Refractive Surgery Today Europe
s CATARACT SURGERY CASE FILES IOL DISLOCATION AFTER A FALL With one haptic in the anterior chamber, how would these surgeons proceed? BY AUDREY R. TALLEY ROSTOV, MD; QUENTIN B. ALLEN, MD; S. ASHA BALAKRISHNAN, MD; ALAN S. CRANDALL, MD; H. BURKHARD DICK, MD, PHD, FEBOS-CR; AND ALANNA NATTIS, DO, FAAO CASE PRESENTATION A 73-year-old woman presents with a complaint of decreased vision in her right eye since a fall several weeks ago. The patient underwent uneventful cataract surgery approximately 10 years ago. BCVA is 20/200 OD. A slit-lamp examination reveals inferior corneal edema and a dislocated three-piece IOL (Figure). The inferior haptic and the inferior portion of the optic are located anterior to the iris. Some heme is visible at the inferior border of the pupil. The cornea and sclera are intact, and a dilated fundus examination and B-scan ultrasound of the retina show no retinal detachment. How would you proceed? —Case prepared by Audrey R. Talley Rostov, MD Figure. The inferior haptic of a three-piece IOL is located in the anterior chamber. the other haptic is also in the capsular bag, I would attempt to remove it from the bag after viscodissection for repositioning in the sulcus, because returning the IOL to the bag at that QUENTIN B. ALLEN, MD point would typically be impossible. S. ASHA BALAKRISHNAN, MD Moreover, trauma might have Obviously the IOL should be significantly weakened the zonular Based on the level of trauma, repositioned. The real issue is whether or apparatus such that the bag may not repositioning and scleral fixation of not there is an intact zonular structure be intact or able to withstand sulcus the IOL are advisable. -

An Enriching Experience Dealing with Small Pupils
World Ophthalmology News World Ophthalmology Congress 2012 | 16 – 20 February | Abu Dhabi Tackling ROP Therapies for AMD Tears and Bubbles The KIDROP-experience shows that Noumerous new medical therapies for Femtosecond laser flap complications tele-ophthalmology is a cost effective wet age-related macular degeneration are very rare and often linked to the possibility to spread ROP-screening are under investigation. This is one of surgical technique. Usually they can programmes into the ural areas of the most rapidly evolving fields in oph- be handled without consequences for India. ( Page 3 thalmology. ( Page 8 the patient. ( Page 11 An Enriching Experience The World Ophthalmology Congress 2012 in Abu Dhabi: Outstanding Speakers, Ultra-Modern Exhibition Centre, Traditional Hospitality ABU DHABI [jp] – For the first time the heritage, Abu Dhabi promises to be an World Ophthalmology Congress (WOC) ideal setting for scientific communi- will be hosted in the Middle East and cation and innovation.” Africa Region. See, Hear and Taste the Culture he destination is definitely new The local organizers as well as repre- to most of the participants of sentatives of the Abu Dhabi Tourism T ophthalmic congresses: Abu Authority also recognize the congress Dhabi, the capital of the United Arab as an opportunity to present the Emirates (UAE) will be the heart of the capital of the United Arab Emirates ophthalmic world from 16 to 20 and the wider emirate to a worldwide February 2012. Never before such a audience. Members of the Middle East prestigious international medical Africa Council of Ophthalmology meeting has taken place in the Middle (MEACO) strive to ensure that all WOC East and Africa Region. -

Agarwal International Video Symposium
PRE-DESCEMET’S ENDOTHELIAL KERATOPLASTY (PDEK) AMAR AGARWAL Endothelial keratoplasty (EK) has evolved at a brisk pace and the volume of data accumulated over the past 10 years has demonstrated that all the posterior lamellar techniques of endothelial replacement yield far superior visual, topographic, and tectonic results compared with penetrating keratoplasty.1 We report a novel method of endothelial keratoplasty in which the endothelium and Descemet’smembrane (DM) along with the Pre-Descemet’s layer (Dua’s layer-PDL) is transplanted and term it as Pre-Descemet’s Endothelial Keratoplasty (PDEK). Early evidence to support the existence of a distinct pre-Descemet’s layer of tissue was presented by Dua et al2 in 2007 and followed by a detailed paper wherein evidence is presented to further support the presence of the distinct PDL. In PDEK procedure, this layer is included with the endothelium–DM complex thereby providing additional support to the graft. Presence of this layer with its characteristics of relative rigidity and toughness allows easy intraoperative handling and insertion of the tissue as itdoes not tend to scroll as much as the DM alone. The most popular in ‘DM barring’techniques is the big bubble (BB) method where the BB forms a cleavage plane,leaving the DM bare for the dissection in lamellar keratoplasties. This entails thecreation of Type 2 BB where the bubble forms between the PDL and the DM. InPDEK procedure, Type 1 BB2 is formed which typically lies between the PDL and theresidual corneal stroma; thereby creating a dome of PDL-DM-Endothelial complex above the air bubble. -

Ocular Trauma
Clinical Diagnosis and Management of OCULAR TRAUMA System requirement: • Windows XP or above • Power DVD player (Software) • Windows media player 10.0 version or above Accompanying DVD ROM is playable only in Computer and not in DVD player. Kindly wait for few seconds for DVD to autorun. If it does not autorun then please do the following: • Click on my computer • Click the DVD drive labelled JAYPEE and after opening the drive, kindly double click the file Jaypee Clinical Diagnosis and Management of OCULAR TRAUMA Editors Ashok Garg MS PhD FIAO (Bel) FRSM FAIMS ADM FICA Jose M Ruiz-Moreno MD PhD International and National Gold Medalist Professor of Ophthalmology Chairman and Medical Director Albacete Medical School, University of Garg Eye Institute and Research Centre Castilla La Mancha 235-Model Town, Dabra Chowk Avendia de Almansa, 14 02006, ALBACETE Hisar-125005, India Spain B Shukla MS PhD MAMS FICS T Mark Johnson MD FRCS Director of Research Consultant Vitreo Retinal Surgeon RJN Institute of Ophthalmology National Retina Institute Chandra Bhawan, 1, Jhansi Road Suite 101, 5530 Wisconsin Ave Gwalior-474002, India Chevy Chase 20815, USA Jerome Jean Bovet MD Keiki R Mehta MS DO FRSH FIOS Consultant Ophthalmic Surgeon FMH Chairman and Medical Director Clinique de L’oeil Mehta International Eye Institute and 15, Avenue Du Bois-de-law-Chapelle Colaba Eye Hospital CH-1213, Onex, Switzerland Seaside, 147, Shahid Bhagat Singh Road, Mumbai-400005 Mahipal S Sachdev MD India Chairman and Medical Director Centre for Sight, B-5/24, Safdarjung Enclave Bojan Pajic MD New Delhi-110029, India Chief of the Cornea and Refractive Surgery Department CS Dhull MS PhD FIAO Klinik Pallas Louis Giroud- Professor and Head Str. -
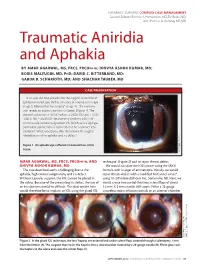
Traumatic Aniridia and Aphakia
CATARACT SURGERY COMPLEX CASE MANAGEMENT Section Editors: Bonnie A. Henderson, MD;Tal Raviv, MD; and Thomas A. Oetting, MS, MD Traumatic Aniridia and Aphakia BY AMAR AGARWAL, MS, FRCS, FRCOPHTH; DHIVYA ASHOK KUMAR, MD; BORIS MALYUGIN, MD, PHD; DAVID C. RITTERBAND, MD; GABOR B. SCHARIOTH, MD; AND SHACHAR TAUBER, MD CASE PRESENTATION A 41-year-old man presents for the surgical correction of aphakia in his left eye. He has a history of trauma to this eye at age 5, followed by “eye surgery” at age 11. The examina- tion reveals an atonic traumatic iris defect (Figure 1). The patient’s refraction is -3.00 D sphere = 20/20 OD and +10.00 -4.00 X 180 = 20/30 OS. Keratometry confirms 4.00 D of with-the-rule corneal astigmatism. His BCVA with a rigid gas permeable contact lens is 20/20 OS, but he is contact lens intolerant. What would you offer this patient for surgical rehabilitation of his aphakia and iris defect? Raviv,MD.) Tal of (Courtesy Figure 1. An aphakic eye suffered a traumatic loss of iris tissue. AMAR AGARWAL, MS, FRCS, FRCOPHTH,AND technique1 (Figure 2) and to repair the iris defect. DHIVYA ASHOK KUMAR, MD We would calculate the IOL’s power using the SRK II The case described seems challenging due to the formula with a target of emmetropia. Initially, we would aphakia, high corneal astigmatism, and iris defect. repair the iris defect with a modified McCannel suture2 Without capsular support, the IOL cannot be placed in using 10–0 Prolene (Ethicon, Inc., Somerville, NJ). -
Glued IOL Scaffold Technique
TACKLING POSTERIOR CAPSULE RUPTURE AND IOL IMPLANTATION: A VIDEO BASED COURSE MONDAY - 20 th APRIL, 2015: 10.00 AM-11.30 AM, SD CC, ROOM 6 F ; SESSION 20-202 COURSE DESCRIPTION BASIC FUNDAMENTALS Early recognition and management of PC rupture is a key to prevent the sequential complications which can trail and have an adverse effect on the visual output. The following signs should be watched upon for early recognition of a PC rupture. • Sudden deepening of anterior chamber with momentary dilation of pupil • Sudden transitory appearance of red reflex • Difficulty in rotating a previously mobile nucleus • Undue tilting of one pole of nucleus …THE COURSE WILL HIGHLIGHT THE METHODS OF HANDLING POSTERIOR CAPSULE (PC) RUPTURE, ITS EFFECTIVE MANAGEMENT AND VARIOUS MODALITIES OF IOL IMPLANTATION. MANAGEMENT PROTOCOL OF PC BASICS RUPTURE AND IOL IMPLANTATION • In the context of a ruptured posterior capsule, the immediate reflex • Lower down phaco parameters action to be deterred on behalf of the surgeon is to withdraw the • Low flow rate phacoemulsification probe suddenly from the eye. A dispersive OVD from the side port incision should be used to plug-in the posterior • Moderate vacuum capsule defect and fill and stabilise the anterior chamber followed by withdrawal of the phacoemulsification probe. • Low to moderate phaco burst energy to promote • The posterior capsule rupture when small is converted into a posterior capsulorhexis with stable margin facilitating the follow ability implantation of a one piece foldable acrylic IOL in the capsular bag. But if the capsular rupture opening is large, sulcus fixation of an IOL is preferable in cases with adequate anterior capsular margin support. -

Glued Posterior Chamber IOL in Eyes with Deficient Capsular Support
Eye (2010) 24, 1143–1148 & 2010 Macmillan Publishers Limited All rights reserved 0950-222X/10 $32.00 www.nature.com/eye Glued posterior DA Kumar, A Agarwal, G Prakash, S Jacob, CLINICAL STUDY Y Saravanan and A Agarwal chamber IOL in eyes with deficient capsular support: a retrospective analysis of 1-year post-operative outcomes Abstract Eye (2010) 24, 1143–1148; doi:10.1038/eye.2010.10; published online 12 February 2010 Purpose To evaluate the post-operative outcome of fibrin glue-assisted posterior Keywords: glued IOL; sutureless IOL; IOL in chamber intraocular lens (IOL) implantation deficient capsule in eyes with deficient capsular support after 1 year. Methods Eyes operated with fibrin glue- assisted posterior chamber IOL implantation Introduction from December 2007 to May 2008 were Intraocular lens (IOL) implantation in eyes with included. The post-operative best spectacle- deficient capsular support has been at the forefront corrected visual acuity (BCVA), uncorrected of surgical research for many years now. IOL visual acuity (UCVA), intraocular pressure implantation in the ciliary sulcus is possible in eyes (IOP), central macular thickness, and specular with adequate anterior capsular support.1 count were evaluated. IOL position and However, in eyes with deficient anterior capsular centration at 1 year was determined. The rim, anterior chamber (AC) IOL, iris claw lenses, or 1-year post-operative complications were sutured scleral-fixated (SF) IOL are usually analysed. performed.2–8 Fibrin glue-assisted posterior Results A total of 53 eyes of 53 patients were chamber (PC) IOL implantation is a new technique analysed. There was significant improvement startedinDecember2007ineyeswithdeficient in UCVA (P ¼ 0.000) and BCVA (P ¼ 0.000). -
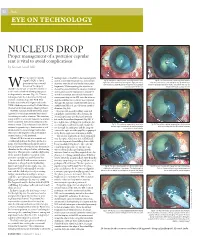
Nucleus Drop After PCR
50 News EYE on TECHnology ProperNUCLEUS management of a posteriorDROP capsular rent is vital to avoid complications by Soosan Jacob MD hen a posterior capsule leading haptic of the IOL to be injected gently rupture (PCR) is noted and in a controlled manner into the anterior Fig 1A: A posterior capsular rent is seen with retained cortex, Fig 1B: The vitrector probe is used to perform anterior epinucleus and a retained nuclear fragment. Flaps seen are for vitrectomy and remove cortex and epinucleus by alternating it prior to nucleus removal, chamber over the iris and under the nuclear later fixation of a glued IOL due to the absence of an adequate between cutting and aspiration modes. The vitrector may also be the aim of the surgery fragments. While injecting, the injector tip capsular support in this case introduced through a corneal incision Wshould be to try and remove the nucleus in should be placed within the anterior chamber a safe manner without allowing any pieces and wound assisted implantation should be to drop into the vitreous (Fig 1A). Various avoided to prevent uncontrolled entry and techniques have been described to try and consequent drop of the IOL into the vitreous. prevent a nucleus drop after PCR. This A globe stabilisation rod may also be placed includes innovative techniques such as the through the side port under the IOL optic to HEMA lifeboat proposed by Dr Keiki Mehta, stabilise the IOL as it unfolds in the anterior phacoemulsification using a Sheet's glide etc. chamber (Fig 2A). The IOL Scaffold described by Prof Amar In cases with a good pupillary tone and Agarwal is a technique recently introduced a pupillary size between 5.0 to 6.0mm, the for utilising in such a situation.