Lecture 3: Magnetization, from Classical to Quantum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basic Magnetic Measurement Methods
Basic magnetic measurement methods Magnetic measurements in nanoelectronics 1. Vibrating sample magnetometry and related methods 2. Magnetooptical methods 3. Other methods Introduction Magnetization is a quantity of interest in many measurements involving spintronic materials ● Biot-Savart law (1820) (Jean-Baptiste Biot (1774-1862), Félix Savart (1791-1841)) Magnetic field (the proper name is magnetic flux density [1]*) of a current carrying piece of conductor is given by: μ 0 I dl̂ ×⃗r − − ⃗ 7 1 - vacuum permeability d B= μ 0=4 π10 Hm 4 π ∣⃗r∣3 ● The unit of the magnetic flux density, Tesla (1 T=1 Wb/m2), as a derive unit of Si must be based on some measurement (force, magnetic resonance) *the alternative name is magnetic induction Introduction Magnetization is a quantity of interest in many measurements involving spintronic materials ● Biot-Savart law (1820) (Jean-Baptiste Biot (1774-1862), Félix Savart (1791-1841)) Magnetic field (the proper name is magnetic flux density [1]*) of a current carrying piece of conductor is given by: μ 0 I dl̂ ×⃗r − − ⃗ 7 1 - vacuum permeability d B= μ 0=4 π10 Hm 4 π ∣⃗r∣3 ● The Physikalisch-Technische Bundesanstalt (German national metrology institute) maintains a unit Tesla in form of coils with coil constant k (ratio of the magnetic flux density to the coil current) determined based on NMR measurements graphics from: http://www.ptb.de/cms/fileadmin/internet/fachabteilungen/abteilung_2/2.5_halbleiterphysik_und_magnetismus/2.51/realization.pdf *the alternative name is magnetic induction Introduction It -

Coey-Slides-1.Pdf
These lectures provide an account of the basic concepts of magneostatics, atomic magnetism and crystal field theory. A short description of the magnetism of the free- electron gas is provided. The special topic of dilute magnetic oxides is treated seperately. Some useful books: • J. M. D. Coey; Magnetism and Magnetic Magnetic Materials. Cambridge University Press (in press) 600 pp [You can order it from Amazon for £ 38]. • Magnétisme I and II, Tremolet de Lachesserie (editor) Presses Universitaires de Grenoble 2000. • Theory of Ferromagnetism, A Aharoni, Oxford University Press 1996 • J. Stohr and H.C. Siegmann, Magnetism, Springer, Berlin 2006, 620 pp. • For history, see utls.fr Basic Concepts in Magnetism J. M. D. Coey School of Physics and CRANN, Trinity College Dublin Ireland. 1. Magnetostatics 2. Magnetism of multi-electron atoms 3. Crystal field 4. Magnetism of the free electron gas 5. Dilute magnetic oxides Comments and corrections please: [email protected] www.tcd.ie/Physics/Magnetism 1 Introduction 2 Magnetostatics 3 Magnetism of the electron 4 The many-electron atom 5 Ferromagnetism 6 Antiferromagnetism and other magnetic order 7 Micromagnetism 8 Nanoscale magnetism 9 Magnetic resonance Available November 2009 10 Experimental methods 11 Magnetic materials 12 Soft magnets 13 Hard magnets 14 Spin electronics and magnetic recording 15 Other topics 1. Magnetostatics 1.1 The beginnings The relation between electric current and magnetic field Discovered by Hans-Christian Øersted, 1820. ∫Bdl = µ0I Ampère’s law 1.2 The magnetic moment Ampère: A magnetic moment m is equivalent to a current loop. Provided the current flows in a plane m = IA units Am2 In general: m = (1/2)∫ r × j(r)d3r where j is the current density; I = j.A so m = 1/2∫ r × Idl = I∫ dA = m Units: Am2 1.3 Magnetization Magnetization M is the local moment density M = δm/δV - it fluctuates wildly on a sub-nanometer and a sub-nanosecond scale. -

Magnetization and Demagnetization Studies of a HTS Bulk in an Iron Core Kévin Berger, Bashar Gony, Bruno Douine, Jean Lévêque
Magnetization and Demagnetization Studies of a HTS Bulk in an Iron Core Kévin Berger, Bashar Gony, Bruno Douine, Jean Lévêque To cite this version: Kévin Berger, Bashar Gony, Bruno Douine, Jean Lévêque. Magnetization and Demagnetization Stud- ies of a HTS Bulk in an Iron Core. IEEE Transactions on Applied Superconductivity, Institute of Electrical and Electronics Engineers, 2016, 26 (4), pp.4700207. 10.1109/TASC.2016.2517628. hal- 01245678 HAL Id: hal-01245678 https://hal.archives-ouvertes.fr/hal-01245678 Submitted on 19 Dec 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1PoBE_12 1 Magnetization and Demagnetization Studies of a HTS Bulk in an Iron Core Kévin Berger, Bashar Gony, Bruno Douine, and Jean Lévêque Abstract—High Temperature Superconductors (HTS) are large quantity, with good and homogeneous properties, they promising materials in variety of practical applications due to are still the most promising materials for the applications of their ability to act as powerful permanent magnets. Thus, in this superconductors. paper, we have studied the influence of some pulsed and There are several ways to magnetize HTS bulks; but we pulsating magnetic fields applied to a magnetized HTS bulk assume that the most convenient one is to realize a method in sample. -

Apparatus for Magnetization and Efficient Demagnetization of Soft
3274 IEEE TRANSACTIONS ON MAGNETICS, VOL. 45, NO. 9, SEPTEMBER 2009 Apparatus for Magnetization and Efficient Demagnetization of Soft Magnetic Materials Paul Oxley Physics Department, The College of the Holy Cross, Worcester, MA 01610 USA This paper describes an electrical circuit that can be used to automatically magnetize and ac-demagnetize moderately soft magnetic materials and with minor modifications could be used to demagnetize harder magnetic materials and magnetic geological samples. The circuit is straightforward to replicate, easy to use, and low in cost. Independent control of the demagnetizing current frequency, am- plitude, and duration is available. The paper describes the circuit operation in detail and shows that it can demagnetize a link-shaped specimen of 430FR stainless steel with 100% efficiency. Measurements of the demagnetization efficiency of the specimen with different ac-demagnetization frequencies are interpreted using eddy-current theory. The experimental results agree closely with the theoretical predictions. Index Terms—Demagnetization, demagnetizer, eddy currents, magnetic measurements, magnetization, residual magnetization. I. INTRODUCTION to magnetize a magnetic sample by delivering a current propor- HERE is a widespread need for a convenient and eco- tional to an input voltage provided by the user and can be used T nomical apparatus that can ac-demagnetize magnetic ma- to measure magnetic properties such as - hysteresis loops terials. It is well known that to accurately measure the mag- and magnetic permeability. netic properties of a material, it must first be in a demagnetized Our apparatus is simple, easy to use, and economical. It state. For this reason, measurements of magnetization curves uses up-to-date electronic components, unlike many previous and hysteresis loops use unmagnetized materials [1], [2] and it designs [9]–[13], which therefore tend to be rather compli- is thought that imprecise demagnetization is a leading cause for cated. -

Quantum Mechanics Magnetization This Article Is About Magnetization As It Appears in Maxwell's Equations of Classical Electrodynamics
Quantum Mechanics_magnetization This article is about magnetization as it appears in Maxwell's equations of classical electrodynamics. For a microscopic description of how magnetic materials react to a magnetic field, see magnetism. For mathematical description of fields surrounding magnets and currents, see magnetic field. In classical Electromagnetism, magnetization [1] ormagnetic polarization is the vector field that expresses the density of permanent or inducedmagnetic dipole moments in a magnetic material. The origin of the magnetic moments responsible for magnetization can be either microscopic electric currents resulting from the motion of electrons inatoms, or the spin of the electrons or the nuclei. Net magnetization results from the response of a material to an external magnetic field, together with any unbalanced magnetic dipole moments that may be inherent in the material itself; for example, inferromagnets. Magnetization is not alwayshomogeneous within a body, but rather varies between different points. Magnetization also describes how a material responds to an appliedmagnetic field as well as the way the material changes the magnetic field, and can be used to calculate the forces that result from those interactions. It can be compared to electric polarization, which is the measure of the corresponding response of a material to an Electric field in Electrostatics. Physicists and engineers define magnetization as the quantity of magnetic moment per unit volume. It is represented by a vector M. Contents 1 Definition 2 Magnetization in Maxwell's equations 2.1 Relations between B, H, and M 2.2 Magnetization current 2.3 Magnetostatics 3 Magnetization dynamics 4 Demagnetization 4.1 Applications of Demagnetization 5 See also 6 Sources Definition Magnetization can be defined according to the following equation: Here, M represents magnetization; m is the vector that defines the magnetic moment; V represents volume; and N is the number of magnetic moments in the sample. -

Demagnetizing Fields and Magnetization Reversal in Permanent Magnets
https://doi.org/10.1016/j.scriptamat.2017.11.020 Searching the weakest link: Demagnetizing fields and magnetization reversal in permanent magnets J. Fischbacher1, A. Kovacs1, L. Exl2,3, J. Kühnel4, E. Mehofer4, H. Sepehri-Amin5, T. Ohkubo5, K. Hono5, T. Schrefl1 1 Center for Integrated Sensor Systems, Danube University Krems, 2700 Wiener Neustadt, Austria 2 Faculty of Physics, University of Vienna, 1090 Vienna, Austria 3 Faculty of Mathematics, University of Vienna, 1090 Vienna, Austria 4 Faculty of Computer Science, University of Vienna, 1090 Vienna, Austria 5 Elements Strategy Initiative Center for Magnetic Materials, National Institute for Materials Science, Tsukuba 305-0047, Japan Abstract Magnetization reversal in permanent magnets occurs by the nucleation and expansion of reversed domains. Micromagnetic theory offers the possibility to localize the spots within the complex structure of the magnet where magnetization reversal starts. We compute maps of the local nucleation field in a Nd2Fe14B permanent magnet using a model order reduction approach. Considering thermal fluctuations in numerical micromagnetics we can also quantify the reduction of the coercive field due to thermal activation. However, the major reduction of the coercive field is caused by the soft magnetic grain boundary phases and misorientation if there is no surface damage. 1. Introduction With the rise of sustainable energy production and eco-friendly transport there is an increasing demand for permanent magnets. The generator of a direct drive wind mill requires high performance magnets of 400 kg/MW power; and on average a hybrid and electric vehicle needs 1.25 kg of high end permanent magnets [1]. Modern high-performance magnets are based on Nd2Fe14B. -

Ch. 2 Magnetostatics Ki-Suk Lee Class Lab
Tue Thur 13:00-14:15 (S103) Ch. 2 Magnetostatics Ki-Suk Lee Class Lab. Materials Science and Engineering Nano Materials Engineering Track Goal of this class Goal of this class Goal of this class Goal of this class Goal of this chapter We begin with magnetostatics, the classical physics of the magnetic fields, forces and energies associated with distributions of magnetic material and steady electric currents. The concepts presented here underpin the magnetism of solids. Magnetostatics refers to situations where there is no time dependence. 2.1 The magnetic dipole moment The elementary quantity in solid-state magnetism is the magnetic moment m. The local magnetization M(r ) fluctuates on an atomic scale – dots represent the atoms. The mesoscopic average, shown by the dashed line, is uniform. the local magnetization M(r,t ) which fluctuates wildly on a subnanometre scale, and also rapidly in time on a subnanosecond scale. But for our purposes, it is more useful to define a mesoscopic average <M(r,t )> over a distance of order a few Nanometres, and times of order a few microseconds to arrive at a steady, homogeneous, local magnetization M(r). The time-averaged magnetic moment δm in a mesoscopic volume δV is 2.1 The magnetic dipole moment Continuous medium approximation The representation of the magnetization of a solid by the quantity M(r) which varies smoothly on a mesoscopic scale. The concept of magnetization of a ferromagnet is often extended to cover the macroscopic average over a sample: According to Amp`ere, a magnet is equivalent to a circulating electric current; the elementary magnetic moment m can be represented by a tiny current loop. -

1 Dipolar Interaction and Demagnetizing Effects In
1 Dipolar interaction and demagnetizing effects in magnetic nanoparticle dispersions: introducing the Mean Field Interacting Superparamagnet Model (MFISP Model) F.H. Sánchez 1*, P. Mendoza Zélis 1,2 , M.L. Arciniegas 1, G.A. Pasquevich 1,2 and M.B. Fernández van Raap 1 1IFLP-CCT- La Plata-CONICET and Departamento de Física, Facultad de Ciencias Exactas, C. C. 67, Universidad Nacional de La Plata, 1900 La Plata, Argentina 2Departamento de Ciencias Básicas, Facultad de Ingeniería, Universidad Nacional de La Plata, 1900 La Plata, Argentina *corresponding autor: [email protected] Abstract A model is developed with the aim of analyzing relevant aspects of interacting magnetic nanoparticles systems (frequently called interacting superparamagnets). Model is built from magnetic dipolar interaction and demagnetizing mean field concepts. By making reasonable simplifying approximations a simple and useful expression for effective demagnetizing factors is achieved, which allows for the analysis of uniform and non-uniform spatial distributions of nanoparticles, in particular for the occurrence of clustering. This expression is a function of demagnetizing factors associated with specimen and clusters shapes, and of the mean distances between near neighbor nanoparticles and between clusters, relative to the characteristic sizes of each of these two types of objects, respectively. It explains effects of magnetic dipolar interactions, such as the observation of apparent nanoparticle magnetic- moments smaller than real ones and approaching zero as temperature decreases. It is shown that by performing a minimum set of experimental determinations along principal directions of geometrically well-defined specimens, model application allows retrieval of nanoparticle intrinsic properties, like mean volume, magnetic moment and susceptibility in the absence of interactions. -

Numerical Micromagnetics : Finite Difference Methods
Numerical Micromagnetics : Finite Difference Methods J. Miltat1 and M. Donahue2 1 Laboratoire de Physique des Solides, Universit´eParis XI Orsay & CNRS, 91405 Orsay, France [email protected] 2 Mathematical & Computational Sciences Division, National Institute of Standards and Technology, Gaithersburg MD 20899-8910, USA [email protected] Key words: micromagnetics, finite differences, boundary conditions, Landau- Lifshitz-Gilbert, magnetization dynamics, approximation order Summary. Micromagnetics is based on the one hand on a continuum approxima- tion of exchange interactions, including boundary conditions, on the other hand on Maxwell equations in the non-propagative (static) limit for the evaluation of the demagnetizing field. The micromagnetic energy is most often restricted to the sum of the exchange, demagnetizing or (self-)magnetostatic, Zeeman and anisotropy en- ergies. When supplemented with a time evolution equation, including field induced magnetization precession, damping and possibly additional torque sources, micro- magnetics allows for a precise description of magnetization distributions within finite bodies both in space and time. Analytical solutions are, however, rarely available. Numerical micromagnetics enables the exploration of complexity in small size mag- netic bodies. Finite difference methods are here applied to numerical micromag- netics in two variants for the description of both exchange interactions/boundary conditions and demagnetizing field evaluation. Accuracy in the time domain is also discussed and a simple tool provided in order to monitor time integration accuracy. A specific example involving large angle precession, domain wall motion as well as vortex/antivortex creation and annihilation allows for a fine comparison between two discretization schemes with as a net result, the necessity for mesh sizes well below the exchange length in order to reach adequate convergence. -
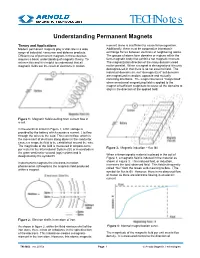
Understanding Permanent Magnets
TECHNotes Understanding Permanent Magnets Theory and Applications moment alone is insufficient to cause ferromagnetism. Modern permanent magnets play a vital role in a wide Additionally, there must be cooperative interatomic range of industrial, consumer and defense products. exchange forces between electrons of neighboring atoms. Efficient use of permanent magnets in these devices The groups of atoms form domains or regions within the requires a basic understanding of magnetic theory. To ferro-magnetic body that exhibit a net magnetic moment. achieve this end it is helpful to understand that all The magnetization direction of the many domains need magnetic fields are the result of electrons in motion. not be parallel. When a magnet is demagnetized it is only demagnetized in that there is no net external field. The individual domains are not “demagnetized” but domains are magnetized in random, opposite and mutually canceling directions. The magnet becomes “magnetized” when an external magnetizing field is applied to the magnet of sufficient magnitude to cause all the domains to align in the direction of the applied field. Figure 1: Magnetic field resulting from current flow in a coil. In the electrical circuit in Figure 1, a DC voltage is provided by the battery which causes a current, I, to flow through the wires to the load. This current flow, which is the movement of electrons along atoms in the conductor, causes a magnetic field to be established around the wire. The magnitude of the field is measured in ampere-turns Figure 3: Magnetic induction – flux (Φ) per meter in the International System (SI) or in oersteds in the gram-centimeter-second (cgs) system and is When a ferromagnetic material is placed in the coil of designated by the symbol H. -

J. M. D. Coey School of Physics and CRANN, Trinity College Dublin Ireland
Fundamentals of Magnetism – 1 J. M. D. Coey School of Physics and CRANN, Trinity College Dublin Ireland. 1. Introduction; basic quantities 2. Magnetic Phenomena. SUMMER SCHOOL 3. Units Comments and corrections please: [email protected] www.tcd.ie/Physics/Magnetism ! Lecture 1 covers basic concepts in magnetism; Firstly magnetic moment, magnetization and the two magnetic fields are presented. Internal and external fields are distinguished. Magnetic energy and forces are discussed. Magnetic phenomena exhibited by functional magnetic materials are briefly presented, and ferromagnetic, ferrimagnetic and antiferromagnetic order introduced. SI units are explained, and dimensions are provided for magnetic, electrical and other physical properties. An elementary knowledge of vector calculus and electromagnetism is assumed. Books Some useful books include: • J. M. D. Coey; Magnetism and Magnetic Magnetic Materials. Cambridge University Press (2010) 614 pp An up to date, comprehensive general text on magnetism. Indispensable! • S. Blundell Magnetism in Condensed Matter, Oxford 2001 A good, readable treatment of the basics. • D. C. Jilles An Introduction to Magnetism and Magnetic Magnetic Materials, Magnetic Sensors and Magnetometers, 3rd edition CRC Press, 2014 480 pp Q & A format. • R. C. O’Handley. Modern Magnetic Magnetic Materials, Wiley, 2000, 740 pp Q & A format. •J. Stohr and H. C. Siegman Magnetism: From fundamentals to nanoscale dynamics Springer 2006, 820 pp. Good for spin transport and magnetization dynamics. Unconventional definition of M • K. M. Krishnan Fundamentals and Applications of Magnetic Material, Oxford, 2017, 816 pp Recent general text.. Good for imaging, nanoparticles and medical applications. IEEE Santander 2017 1 Introduction 2 Magnetostatics 3 Magnetism of the electron 4 The many-electron atom 5 Ferromagnetism 6 Antiferromagnetism and other magnetic order 7 Micromagnetism 8 Nanoscale magnetism 9 Magnetic resonance 10 Experimental methods 11 Magnetic materials 12 Soft magnets 13 Hard magnets 14 Spin electronics and magnetic recording 614 pages. -

Effective Demagnetization Factors of Diamagnetic Samples of Various
Effective Demagnetizing Factors of Diamagnetic Samples of Various Shapes R. Prozorov1, 2, ∗ and V. G. Kogan1, y 1Ames Laboratory, Ames, IA 50011 2Department of Physics & Astronomy, Iowa State University, Ames, IA 50011 (Dated: Submitted: 16 December 2017; accepted in Phys. Rev. Applied: 26 June 2018) Effective demagnetizing factors that connect the sample magnetic moment with the applied mag- netic field are calculated numerically for perfectly diamagnetic samples of various non-ellipsoidal shapes. The procedure is based on calculating total magnetic moment by integrating the magnetic induction obtained from a full three dimensional solution of the Maxwell equations using adaptive mesh. The results are relevant for superconductors (and conductors in AC fields) when the London penetration depth (or the skin depth) is much smaller than the sample size. Simple but reasonably accurate approximate formulas are given for practical shapes including rectangular cuboids, finite cylinders in axial and transverse field as well as infinite rectangular and elliptical cross-section strips. I. INTRODUCTION state to compute the approximate N−factors for a 2D sit- uation of infinitely long strips of rectangular cross-section Correcting results of magnetic measurements for the in perpendicular field and he extended these results to fi- distortion of the magnetic field inside and around a finite nite 3D cylinders (also of rectangular cross-section) in sample of arbitrary shape is not trivial, but necessary the axial magnetic field. We find an excellent agreement part of experimental studies in magnetism and super- between our calculations and Brandt's results for these conductivity. The internal magnetic field is uniform only geometries.