Chemotaxonomy and Pharmacology of Gentianaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Genetićka Transformacija Kićice
UNIVERZITET U BEOGRADU BIOLOŠKI FAKULTET 4 5 5 (Centaurium erythraea Rafn.) AtCKX1 I AtCKX2 GENIMA doktorska disertacija Beograd, 2012 UNIVERSITY OF BELGRADE FACULTY OF BIOLOGY 4 GENETIC TRANSFORMATION OF CENTAURY (Centaurium erythraea Rafn.) USING AtCKX1 AND AtCKX2 GENES Doctoral Dissertation Belgrade, 2012 _________________________________________ 4 6 -a Univerziteta u Beogradu, mentor _________________________________________ 4 4 _________________________________________ 4 6i saradnik IBISS-a Univerziteta u Beogradu Datum odbrane: ________________ 7 istraživanja » 4« Univerziteta u Beogradu. Zahvaljujem se svom m 4 6 4 savetima, razumevanju, optimizmu i podršci koju mi je pružala tokom svih ovih godina. ! " # 4 4 da mi pomogne u analizi i prezentaciji rezultata. Posebnu zahvalnost dugujem dr Ani 4 46 teksta. Deo istraživanja o 6 " " " 7 5 % prilikom želim da se zahvalim dr Václav Motyka koji je to 4 7 analizi rezultata. Neizmernu zahvalnost dugujem mr Aleksandru Cingelu i Martinu Rasporu koji su 64 " primenjene u ovoj disertaciji. ' 6" 6 ( 4 kojoj se ovom prilikom 6zahvaljujem. Hemijska ispitivanja sekundarnih metabolita, HPLC analizom sekoiridoida i # ) 4-( 4*+" 4 rezultata 6ja teksta ove disertacije. Izolaciju ksantona iz biljnog materijala, 7 " , 4 na Hemijskom fakultetu Univerziteta u Beogradu i ovom prilikom joj se najtoplije zahvaljujem. Veliko hvala -

Native Or Suitable Plants City of Mccall
Native or Suitable Plants City of McCall The following list of plants is presented to assist the developer, business owner, or homeowner in selecting plants for landscaping. The list is by no means complete, but is a recommended selection of plants which are either native or have been successfully introduced to our area. Successful landscaping, however, requires much more than just the selection of plants. Unless you have some experience, it is suggested than you employ the services of a trained or otherwise experienced landscaper, arborist, or forester. For best results it is recommended that careful consideration be made in purchasing the plants from the local nurseries (i.e. Cascade, McCall, and New Meadows). Plants brought in from the Treasure Valley may not survive our local weather conditions, microsites, and higher elevations. Timing can also be a serious consideration as the plants may have already broken dormancy and can be damaged by our late frosts. Appendix B SELECTED IDAHO NATIVE PLANTS SUITABLE FOR VALLEY COUNTY GROWING CONDITIONS Trees & Shrubs Acer circinatum (Vine Maple). Shrub or small tree 15-20' tall, Pacific Northwest native. Bright scarlet-orange fall foliage. Excellent ornamental. Alnus incana (Mountain Alder). A large shrub, useful for mid to high elevation riparian plantings. Good plant for stream bank shelter and stabilization. Nitrogen fixing root system. Alnus sinuata (Sitka Alder). A shrub, 6-1 5' tall. Grows well on moist slopes or stream banks. Excellent shrub for erosion control and riparian restoration. Nitrogen fixing root system. Amelanchier alnifolia (Serviceberry). One of the earlier shrubs to blossom out in the spring. -
Dwarf Thistle, Cirsi
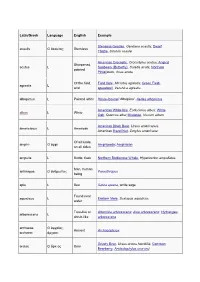
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

Amorpha Canescens Pursh Leadplant
leadplant, Page 1 Amorpha canescens Pursh leadplant State Distribution Best Survey Period Photo by Susan R. Crispin Jan Feb Mar Apr May Jun Jul Aug Sept Oct Nov Dec Status: State special concern the Mississippi valley through Arkansas to Texas and in the western Great Plains from Montana south Global and state rank: G5/S3 through Wyoming and Colorado to New Mexico. It is considered rare in Arkansas and Wyoming and is known Other common names: lead-plant, downy indigobush only from historical records in Montana and Ontario (NatureServe 2006). Family: Fabaceae (pea family); also known as the Leguminosae. State distribution: Of Michigan’s more than 50 occurrences of this prairie species, the vast majority of Synonym: Amorpha brachycarpa E.J. Palmer sites are concentrated in southwest Lower Michigan, with Kalamazoo, St. Joseph, and Cass counties alone Taxonomy: The Fabaceae is divided into three well accounting for more than 40 of these records. Single known and distinct subfamilies, the Mimosoideae, outlying occurrences have been documented in the Caesalpinioideae, and Papilionoideae, which are last two decades from prairie remnants in Oakland and frequently recognized at the rank of family (the Livingston counties in southeast Michigan. Mimosaceae, Caesalpiniaceae, and Papilionaceae or Fabaceae, respectively). Of the three subfamilies, Recognition: Leadplant is an erect, simple to sparsely Amorpha is placed within the Papilionoideae (Voss branching shrub ranging up to ca. 1 m in height, 1985). Sparsely hairy plants of leadplant with greener characterized by its pale to grayish color derived from leaves have been segregated variously as A. canescens a close pubescence of whitish hairs that cover the plant var. -

Shale Hollow Preserve Bio Blitz May 15-16, 2015
Shale Hollow Preserve Bio Blitz May 15-16, 2015 SCIENTIFIC NAME COMMON NAME Terrestrial Insect Vanessa atalanta Red Admiral Cicindela sexguttata Tiger Beetle Malacosoma americanum Eastern Tent Caterpillar Pieris rapae Cabbage White Lycaena hyllus Bronze Copper Papilio glaucus Eastern Tiger Swallowtail Epargereus clarus Silver-spotted Skipper Cicindela sexguttata Six-spotted Tiger Beetle Phyciodes tharos Pearl Crescent Nicrophorus orbicollis Roundneck Sexton Beetle Aquatic Macroinvertebrates Allocapnia spp. Stonefly Nymph Aquatic Invertebrates Sphaeriidae spp. Fingernail Clam Phreatoious spp. Freshwater Isopod Limnephilus spp. Northern Caddisfly Mammals Sciurus carolinensis Eastern Gray Squirrel Sciurus niger Fox Squirrel Microtus pennsylvanicus Meadow Vole Odocoileus virginianus White-tailed Deer Tamias striatus Eastern Chipmunk Amphibians Anaxyrus americanus American Toad Desmognathus fuscus Dusky Salamander Hyla versicolor Gray Tree Frog Lithobates clamitans Green Frog Eurycea bislineata Northern Two - lined Salamander Plethodon cinereus Red-backed Salamander Woody Plants Cornus alternifolia Alternate-leaved Dogwood Cornus florida Flowering Dogwood Gaylussacia baccata Black Huckleberry Prunus serotina Black Cherry Rosa multiflora Multiflora Rose Lonicera spp. Bush Honey Suckle Acer sacchrum Sugar Maple Ulmus americana American elm Ligustrum vulgare Common privet Berberis vulgaris European barberry Smilax spp. Greenbrier Lendara benzoin Common Spicebush Viburnum lentago Nannyberry Viburnum Rubus idaeus Red Rasberry Hamamelis virginiana -

Rare Plant Survey of San Juan Public Lands, Colorado
Rare Plant Survey of San Juan Public Lands, Colorado 2005 Prepared by Colorado Natural Heritage Program 254 General Services Building Colorado State University Fort Collins CO 80523 Rare Plant Survey of San Juan Public Lands, Colorado 2005 Prepared by Peggy Lyon and Julia Hanson Colorado Natural Heritage Program 254 General Services Building Colorado State University Fort Collins CO 80523 December 2005 Cover: Imperiled (G1 and G2) plants of the San Juan Public Lands, top left to bottom right: Lesquerella pruinosa, Draba graminea, Cryptantha gypsophila, Machaeranthera coloradoensis, Astragalus naturitensis, Physaria pulvinata, Ipomopsis polyantha, Townsendia glabella, Townsendia rothrockii. Executive Summary This survey was a continuation of several years of rare plant survey on San Juan Public Lands. Funding for the project was provided by San Juan National Forest and the San Juan Resource Area of the Bureau of Land Management. Previous rare plant surveys on San Juan Public Lands by CNHP were conducted in conjunction with county wide surveys of La Plata, Archuleta, San Juan and San Miguel counties, with partial funding from Great Outdoors Colorado (GOCO); and in 2004, public lands only in Dolores and Montezuma counties, funded entirely by the San Juan Public Lands. Funding for 2005 was again provided by San Juan Public Lands. The primary emphases for field work in 2005 were: 1. revisit and update information on rare plant occurrences of agency sensitive species in the Colorado Natural Heritage Program (CNHP) database that were last observed prior to 2000, in order to have the most current information available for informing the revision of the Resource Management Plan for the San Juan Public Lands (BLM and San Juan National Forest); 2. -

Durham E-Theses
Durham E-Theses Ecological Changes in the British Flora WALKER, KEVIN,JOHN How to cite: WALKER, KEVIN,JOHN (2009) Ecological Changes in the British Flora, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/121/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk Ecological Changes in the British Flora Kevin John Walker B.Sc., M.Sc. School of Biological and Biomedical Sciences University of Durham 2009 This thesis is submitted in candidature for the degree of Doctor of Philosophy Dedicated to Terry C. E. Wells (1935-2008) With thanks for the help and encouragement so generously given over the last ten years Plate 1 Pulsatilla vulgaris , Barnack Hills and Holes, Northamptonshire Photo: K.J. Walker Contents ii Contents List of tables vi List of figures viii List of plates x Declaration xi Abstract xii 1. -

In Vitro Regeneration and Transformation of Blackstonia Perfoliata
BIOLOGIA PLANTARUM 48 (3): 333-338, 2004 In vitro regeneration and transformation of Blackstonia perfoliata A. BIJELOVIĆ*1, N. ROSIĆ**, J. MILJUŠ-DJUKIĆ***, S. NINKOVIĆ** and D. GRUBIŠIĆ** Institute of Botany, Faculty of Biology, Takovska 43, 11000 Belgrade, Serbia and Montenegro* Institute for Biological Research “Siniša Stanković”, 29. Novembra 142, 11060 Belgrade, Serbia and Montenegro** Institute for Molecular Genetics and Genetic Engineering, Vojvode Stepe 444a, 11000 Belgrade, Serbia and Montenegro*** Abstract In vitro root culture of yellow wort (Blackstonia perfoliata (L.) Huds.) was initiated on Murashige and Skoog (MS) medium. In the presence of benzylaminopurine (BAP) numerous adventitious buds formed, which developed into shoots. Presence of indole-3-butyric acid (IBA) in media significantly decreased number of buds, but increased development of lateral roots. On hormone-free medium shoots successfully rooted and developed flowers and viable seeds that formed another generation. Shoot cultures of B. perfoliata inoculated with suspension of Agrobacterium rhizogenes strain A4M70GUS developed hairy roots at 3 weeks and they were cultured on hormone-free MS medium. Spontaneous shoot regeneration occurred in 3 clones. Additional key words: Agrobacterium rhizogenes, hairy roots, regeneration, root culture. Introduction Blackstonia perfoliata (yellow wort) (L.) Huds. (Chlora 1997a, Menković et al. 1998, Vintehalter and Vinterhalter perfoliata L., Gentiana perfoliata L., Seguiera perfoliata 1998, Mikula and Rybczynski 2001). O. Kuntze), Gentianaceae, is an annual plant, 10 - 60 cm Since B. perfoliata could be used in medicine instead high, with long internodes, triangular leaves, sometimes of Radix Gentianae, this plant can be produced in great narrowing towards the base (Jovanović-Dunjić 1973). It biomass in culture in vitro. -

Illustrated Flora of East Texas Illustrated Flora of East Texas
ILLUSTRATED FLORA OF EAST TEXAS ILLUSTRATED FLORA OF EAST TEXAS IS PUBLISHED WITH THE SUPPORT OF: MAJOR BENEFACTORS: DAVID GIBSON AND WILL CRENSHAW DISCOVERY FUND U.S. FISH AND WILDLIFE FOUNDATION (NATIONAL PARK SERVICE, USDA FOREST SERVICE) TEXAS PARKS AND WILDLIFE DEPARTMENT SCOTT AND STUART GENTLING BENEFACTORS: NEW DOROTHEA L. LEONHARDT FOUNDATION (ANDREA C. HARKINS) TEMPLE-INLAND FOUNDATION SUMMERLEE FOUNDATION AMON G. CARTER FOUNDATION ROBERT J. O’KENNON PEG & BEN KEITH DORA & GORDON SYLVESTER DAVID & SUE NIVENS NATIVE PLANT SOCIETY OF TEXAS DAVID & MARGARET BAMBERGER GORDON MAY & KAREN WILLIAMSON JACOB & TERESE HERSHEY FOUNDATION INSTITUTIONAL SUPPORT: AUSTIN COLLEGE BOTANICAL RESEARCH INSTITUTE OF TEXAS SID RICHARDSON CAREER DEVELOPMENT FUND OF AUSTIN COLLEGE II OTHER CONTRIBUTORS: ALLDREDGE, LINDA & JACK HOLLEMAN, W.B. PETRUS, ELAINE J. BATTERBAE, SUSAN ROBERTS HOLT, JEAN & DUNCAN PRITCHETT, MARY H. BECK, NELL HUBER, MARY MAUD PRICE, DIANE BECKELMAN, SARA HUDSON, JIM & YONIE PRUESS, WARREN W. BENDER, LYNNE HULTMARK, GORDON & SARAH ROACH, ELIZABETH M. & ALLEN BIBB, NATHAN & BETTIE HUSTON, MELIA ROEBUCK, RICK & VICKI BOSWORTH, TONY JACOBS, BONNIE & LOUIS ROGNLIE, GLORIA & ERIC BOTTONE, LAURA BURKS JAMES, ROI & DEANNA ROUSH, LUCY BROWN, LARRY E. JEFFORDS, RUSSELL M. ROWE, BRIAN BRUSER, III, MR. & MRS. HENRY JOHN, SUE & PHIL ROZELL, JIMMY BURT, HELEN W. JONES, MARY LOU SANDLIN, MIKE CAMPBELL, KATHERINE & CHARLES KAHLE, GAIL SANDLIN, MR. & MRS. WILLIAM CARR, WILLIAM R. KARGES, JOANN SATTERWHITE, BEN CLARY, KAREN KEITH, ELIZABETH & ERIC SCHOENFELD, CARL COCHRAN, JOYCE LANEY, ELEANOR W. SCHULTZE, BETTY DAHLBERG, WALTER G. LAUGHLIN, DR. JAMES E. SCHULZE, PETER & HELEN DALLAS CHAPTER-NPSOT LECHE, BEVERLY SENNHAUSER, KELLY S. DAMEWOOD, LOGAN & ELEANOR LEWIS, PATRICIA SERLING, STEVEN DAMUTH, STEVEN LIGGIO, JOE SHANNON, LEILA HOUSEMAN DAVIS, ELLEN D. -

Current Tracking List
Nevada Division of Natural Heritage Department of Conservation and Natural Resources 901 S. Stewart Street, Suite 5002, Carson City, Nevada 89701-5245 voice: (775) 684-2900 | fax: (775) 684-2909 | web: heritage.nv.gov At-Risk Plant and Animal Tracking List July 2021 The Nevada Division of Natural Heritage (NDNH) A separate list, the Plant and Animal Watch List, systematically curates information on Nevada's contains taxa that could become at-risk in the future. endangered, threatened, sensitive, rare, and at-risk plants and animals providing the most comprehensive Taxa on the At-Risk Plant and Animal Tracking List are source of information on Nevada’s imperiled organized by taxonomic group, and presented biodiversity. alphabetically by scientific name within each group. Currently, there are 639 Tracking List taxa: 285 plants, Nevada's health and economic well-being depend 209 invertebrates, 65 fishes, 9 amphibians, 7 reptiles, upon its biodiversity and wise land stewardship. This 27 birds, and 37 mammals. challenge increases as population and land-use pressures continue to grow. Nevada is among the top Documentation of population status, locations, or 10 states for both the diversity and the vulnerability of other updates or corrections for any of the taxa on its living heritage. With early planning and responsible this list are always welcome. Literature citations with development, economic growth and our biological taxonomic revisions and descriptions of new taxa are resources can coexist. NDNH is a central source for also appreciated. The Nevada Native Species Site information critical to achieving this balance. Survey Report form is available on our website under Management priorities for the state’s imperiled the Submit Data tab and is the preferred format for biodiversity are continually assessed, providing submitting information to NDNH. -

Un Plan De Conservation Pour La Petite Centaurée Vivace En Basse
Un plan de conservation pour la petite Centaurée vivace (Centaurium portense (Brot.) Butcher) bref En en Basse-Normandie Juliette WAYMEL Conservatoire botanique national de Brest (antenne Basse-Normandie) [email protected] Référence bibliographique de l’article : WAYMEL J., 2016 - Un plan de conservation pour la petite Centaurée vivace (Centaurium portense (Brot.) Butcher) en Basse-Normandie. E.R.I.C.A., 30 : 59-67. Résumé : la petite Centaurée vivace (Centaurium portense (Brot.) Butcher) est une espèce endé- mique d’Europe de l’ouest. Ses populations françaises situées en Bretagne et en Basse-Normandie régressent. Protégée en France, cette espèce bénéficie de mesures de conservation. Entre 2013 et 2015, dans le cadre de la rédaction d’un plan de conservation, un bilan de la situation des stations de la Hague (Manche) a été établi et des actions de conservation proposées. Mots clés : Centaurium scilloides ; plan de conservation ; suivi ; Hague. Keywords : Centaurium scilloides ; conservation plan ; monitoring ; Hague. Introduction Pour des besoins de travaux indispensables au maintien des conditions de sécurité du Centre de stockage de déchets radioactifs de la Manche (CSM), l’ANDRA (Agence nationale pour la gestion des déchets radioactifs) a été autorisée par arrêté préfectoral du 15 janvier 2013 à détruire sur envi- ron 1 200 m² plusieurs milliers de pieds de la petite Centaurée vivace (Centaurium portense, connue aussi sous le nom de C. scilloides), espèce protégée en France. Dans le cadre de compensations liées aux travaux, l’antenne de Basse-Normandie du Conservatoire botanique national (CBN) de Brest a été missionnée pour réaliser un plan de conservation des populations de l’espèce sur l’ensemble du cap de la Hague dans la Manche (Waymel et al., à paraître).