Novel Immune Checkpoint Targets: Moving Beyond PD-1 and CTLA-4 Shuang Qin1, Linping Xu2, Ming Yi1, Shengnan Yu1, Kongming Wu1,2* and Suxia Luo2*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(PDCO) Minutes of the Meeting on 29 January - 01 February 2019
1 March 2019 EMA/PDCO/56017/2019 Inspections, Human Medicines Pharmacovigilance and Committees Division Paediatric Committee (PDCO) Minutes of the meeting on 29 January - 01 February 2019 Chair: Dirk Mentzer – Vice-Chair: Koenraad Norga 29 January 2019, 14:00- 19:00, room 3A 30 January 2019, 08:30- 19:00, room 3A 31 January 2019, 08:30- 19:00, room 3A 01 February 2019, 08:30- 13:00, room 3A Disclaimers Some of the information contained in this set of minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the PDCO Committee meeting reports (after the PDCO Opinion is adopted), and on the Opinions and decisions on paediatric investigation plans webpage (after the EMA Decision is issued). Of note, this set of minutes is a working document primarily designed for PDCO members and the work the Committee undertakes. Further information with relevant explanatory notes can be found at the end of this document. Note on access to documents Some documents mentioned in the minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on-going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006). -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Comparative Safety and Efficacy of Anti-PD-1 Monotherapy, Chemotherapy
Lv et al. Journal for ImmunoTherapy of Cancer (2019) 7:159 https://doi.org/10.1186/s40425-019-0636-7 COMMENTARY Open Access Comparative safety and efficacy of anti-PD- 1 monotherapy, chemotherapy alone, and their combination therapy in advanced nasopharyngeal carcinoma: findings from recent advances in landmark trials Jia-Wei Lv1†, Jun-Yan Li1†, Lin-Na Luo2†, Zi-Xian Wang2* and Yu-Pei Chen1* Abstract Recent phase 1–2 trials reported manageable safety profiles and promising antitumor activities of anti-PD-1 drugs (pembrolizumab, nivolumab, camrelizumab and JS001) with/without chemotherapy in recurrent/metastatic nasopharyngeal carcinoma (RM-NPC), however head-to-head comparison among these regimens is lacking. We aimed to comprehensively compare the efficacy and safety of different anti-PD-1 drugs, standard chemotherapy, and their combination therapy in RM-NPC. Adverse event (AE) and objective response rate (ORR) were assessed. The pooled incidence rates of grade 1–5/3–5 AEs were 74.1%/29.6, 54.2%/17.4, 92.3%/24.5, 96.8%/16.1, 91.2%/42.8, and 100%/87.9% for pembrolizumab, nivolumab, JS001, camrelizumab, chemotherapy and camrelizumab+chemotherapy, respectively, which suggested that nivolumab and pembrolizumab exhibited the optimal safety regarding grade 1–5 AEs whereas camrelizumab and nivolumab regarding grade 3–5 AEs. As second- or later-line therapy, ORR was higher with camrelizumab (34.1%), followed by pembrolizumab (26.3%), JS001 (23.3%), and nivolumab (19.0%); whereas ORR with first-line nivolumab reached 40%. Additionally, first-line camrelizumab+chemotherapy achieved a dramatically higher ORR than that with chemotherapy alone (90.9% vs. -

Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions
Review Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions Shilpa Gupta 1,†, David Gill 2,†, Austin Poole 2 and Neeraj Agarwal 2,* 1 Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA; [email protected] 2 Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA; [email protected] (D.G.); [email protected] (A.P.) * Correspondence: [email protected]; Tel.: +1-801-213-5658; Fax: +1-801-585-0124 † These authors contributed equally to this work. Academic Editor: Vita Golubovskaya Received: 14 December 2016; Accepted: 18 January 2017; Published: 27 January 2017 Abstract: Urothelial cancer of the bladder, renal pelvis, ureter, and other urinary organs is the fifth most common cancer in the United States, and systemic platinum-based chemotherapy remains the standard of care for first-line treatment of advanced/metastatic urothelial carcinoma (UC). Until recently, there were very limited options for patients who are refractory to chemotherapy, or do not tolerate chemotherapy due to toxicities and overall outcomes have remained very poor. While the role of immunotherapy was first established in non-muscle invasive bladder cancer in the 1970s, no systemic immunotherapy was approved for advanced disease until the recent approval of a programmed death ligand-1 (PD-L1) inhibitor, atezolizumab, in patients with advanced/metastatic UC who have progressed on platinum-containing regimens. This represents a significant milestone in this disease after a void of over 30 years. In addition to atezolizumab, a variety of checkpoint inhibitors have shown a significant activity in advanced/metastatic urothelial carcinoma and are expected to gain Food and Drug Administration (FDA) approval in the near future. -

LAG-3: from Molecular Functions to Clinical Applications
Open access Review J Immunother Cancer: first published as 10.1136/jitc-2020-001014 on 13 September 2020. Downloaded from LAG-3: from molecular functions to clinical applications Takumi Maruhashi , Daisuke Sugiura , Il- mi Okazaki , Taku Okazaki To cite: Maruhashi T, Sugiura D, ABSTRACT (PD-1) and cytotoxic T lymphocyte antigen Okazaki I, et al. LAG-3: from To prevent the destruction of tissues owing to excessive 4 (CTLA-4) significantly improved the molecular functions to clinical and/or inappropriate immune responses, immune outcomes of patients with diverse cancer applications. Journal for cells are under strict check by various regulatory ImmunoTherapy of Cancer types, revolutionizing cancer treatment. The mechanisms at multiple points. Inhibitory coreceptors, 2020;8:e001014. doi:10.1136/ success of these therapies verified that inhib- including programmed cell death 1 (PD-1) and cytotoxic jitc-2020-001014 itory coreceptors serve as critical checkpoints T lymphocyte antigen 4 (CTLA-4), serve as critical checkpoints in restricting immune responses against for immune cells to not attack the tumor Accepted 29 July 2020 self- tissues and tumor cells. Immune checkpoint inhibitors cells as well as self-tissues. However, response that block PD-1 and CTLA-4 pathways significantly rates are typically lower and immune-related improved the outcomes of patients with diverse cancer adverse events (irAEs) are also observed in types and have revolutionized cancer treatment. However, patients administered with immune check- response rates to such therapies are rather limited, and point inhibitors. This is indicative of the immune-rela ted adverse events are also observed in a continued need to decipher the complex substantial patient population, leading to the urgent need biology of inhibitory coreceptors to increase for novel therapeutics with higher efficacy and lower response rates and prevent such unwanted toxicity. -

9.0 Bn 23 000 200 +
42 | Novartis Annual Report 2017 Innovation The Novartis Institutes for BioMedical Research works in concert with our Global Drug Development group to bring innovative treatments to patients around the world. In 2017, we advanced our drug discovery and development efforts by encouraging greater collaboration and out-of-the-box thinking, exploring new approaches that could improve how we work, and investing in promising tools and technologies. We made progress in priority disease areas with high unmet medical needs. We also marked several key milestones, including US FDA approval – the first of its kind – for a type of personalized cell therapy that could change the course of cancer care. 9.0 bn 23 000 200 + Research and development Scientists, physicians and business Projects in clinical development spending in 2017, amounting to professionals working in research 18.3% of net sales (USD) and development worldwide Collaborative Efficient, effective Progress in important science drug development disease areas We are increasing collaboration We are using digital technology We highlight areas of our work where in research as we try to leverage and data analysis to make drug we are driving significant innovation, innovation from a variety of sources. development swifter and more or where we can potentially have an We are also exploring ways to effective. And we are taking steps important impact on patients and harness digital technology in drug to strengthen our pipeline. public health. discovery, as well as new therapeutic k page 46 k Immuno-oncology page 48 approaches such as cell therapies. k Multiple sclerosis page 50 k page 43 k Liver disease page 52 k Ophthalmology page 53 k Asthma page 55 k Malaria page 56 INNOVATION Novartis Annual Report 2017 | 43 Discovery For new treatments, the journey from laboratory to patient Promoting open innovation starts in the discovery phase where researchers try to Our efforts to increase the flow of ideas between identify potentially groundbreaking therapies. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma
King’s Research Portal DOI: 10.3389/fimmu.2019.00453 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Khair, D. O., Bax, H. J., Mele, S., Crescioli, S., Pellizzari, G., Khiabany, A., Nakamura, M., Harris, R. J., French, E., Hoffmann, R. M., Williams, I. P., Cheung, K. K. A., Thair, B., Beales, C. T., Touizer, E., Signell, A. W., Tasnova, N. L., Spicer, J. F., Josephs, D. H., ... Karagiannis, S. N. (2019). Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma. Frontiers in Immunology , (MAR), [453]. https://doi.org/10.3389/fimmu.2019.00453 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. -

Of Eftilagimod Alpha
Initial results from a Phase II study (TACTI-002) of eftilagimod alpha (soluble LAG-3 Presentation Number: 927P protein) and pembrolizumab as 2nd line treatment for PD-L1 unselected metastatic head and neck cancer (HNSCC) patients 1 2 3 4 5 6 7 Authors: Forster M ; Felip E ; Doger B ; Pousa P ; Carcereny E , Bajaj P ; Church M , Peguero 2, J8, Roxburgh P9, Triebel F10 Trial Design Part C stage 1 – PD-X naive 2nd line HNSCC PD-L1 all comer Affiliates: Part A: 1st line, PD-X naïve NSCLC; Stage IIIB not amenable to curative treatment or stage IV not amenable • 18 patients enrolled, treated and evaluated (16 with ≥ 1 1. Forster: UCL Cancer Institute / University College London Hospitals NHS Foundation, London, UK Baseline Parameters (n=18) N (%) 2. Felip: Vall d’Hebron University Hospital, Barcelona, Spain to EGFR/ALK based therapy, treatment-naïve for advanced/metastatic disease post baseline scan) 3. Doger: START Madrid- Fundación Jiménez Diaz, Madrid, Spain Part B: 2nd line, PD-X refractory NSCLC; Pts after failure of 1st line therapy for metastatic disease which incl. • Different types of HNSCC: Age [yrs] Median 66 (48-78) 4. Lopez Pousa : Hospital de la Santa Creu i Sant Pau, Barcelona, Spain at least 2 cycles of PD-X o 5. Carcereny: Catalan Institute of Oncology Badalona-Hospital Germans Trias i Pujol, B-ARGO group; Badalona, Spain Oropharynx n=6; 33.3 % Female / Male 1 (5.6) / 17 (94.4) 6. Bajaj: Griffith University, Gold Coast, Australia Part C: 2nd line PD-X naive HNSCC; Recurrent disease not amenable to curative treatment, or metastatic o Hypopharynx n=5; 27.8 % ECOG 0 / 1 10 (55.6) / 8 (44.4) 7. -
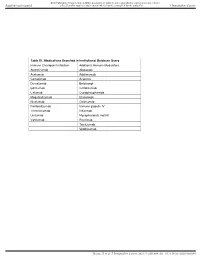
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

Pembrolizumab Monotherapy in Patients with Previously Treated Metastatic High-Grade Neuroendocrine Neoplasms: Joint Analysis of Two Prospective, Non-Randomised Trials
www.nature.com/bjc ARTICLE Clinical Study Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials Namrata Vijayvergia1, Arvind Dasari2, Mengying Deng1, Samuel Litwin1, Taymeyah Al-Toubah3, R. Katherine Alpaugh1, Efrat Dotan1, Michael J. Hall1, Nicole M. Ross1, Melissa M. Runyen1, Crystal S. Denlinger1, Daniel M. Halperin2, Steven J. Cohen4, Paul F. Engstrom1 and Jonathan R. Strosberg3 BACKGROUND: Metastatic high-grade neuroendocrine neoplasms (G3NENs) have limited treatment options after progression on platinum-based therapy. We addressed the role of Pembrolizumab in patients with previously treated metastatic G3NENs. METHODS: Two open-label, phase 2 studies enrolled patients with G3NEN (Ki-67 > 20%) to receive Pembrolizumab at 200 mg I.V. every 3 weeks. Radiographic evaluation was conducted every 9 weeks with overall response rate as the primary endpoint. RESULTS: Between November 2016 and May 2018, 29 patients (13 males/16 females) with G3NENs were enrolled. One patient (3.4%) had an objective response and an additional six patients (20.7%) had stable disease, resulting in a disease control rate of 24.1%. Disease control rate (DCR) at 18 weeks was 10.3% (3/29). There was no difference in the DCR, PFS or OS between the PD-L1- negative and -positive groups (p 0.56, 0.88 and 0.55, respectively). Pembrolizumab was well tolerated with only 9 grade 3, and no grade 4 events considered drug-related. CONCLUSIONS: Pembrolizumab can be safely administered to patients with G3NENs but has limited activity as a single agent. Successful completion of our trials suggest studies in G3NENs are feasible and present an unmet need. -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors