Species Composition, Abundance, and Vertical Distribution of the Stomiid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Sardinella
A CHECK.LIST OF THE FISHES OF INDIA, BURMA AND CEYLON. PART II. CLUPEIFORMES, BATHYCLUPEIFORMES, GALAXIIFORMES, SCOPELIFORMES AND ATELEOPIl~"ORMES. By K. S. l\iISRA, D.Se., F.Z.S., ,,1ssistant Superintendent, Zoological Survey of India, Kaiser Castle, Banaras Gantt. CONTENTS. PAGE. INTRODUOTION •• 382 SYSTEMATIC ACCOUNT 382 Class TELEOSTOMI 382 Subclass ACTINOPTERYGII 382 Order CLUPEIFOR)IES (ISOSPONDYLI, MALACOPTERYGII S. STR., 382 THRISSO)IORPHI). Suborder CLUPEOIDEI .. 382 Superfamily ELOPOIDAE 382 Family ELOPIDAE 382 Elopa 8aurU8 L. 382 Family MEGALOPIDA.E 383 M egalopa cyprinoides (Brouss.) 383 Su perfamily ALBULOIDAE 383 Family ALBULIDAE 383 Albula vulpe8 (L.) 384 Superfamily CLUPEOIDAE ... 384 Family CL UPEIDAE .' 384 SU bfamily DU88umieriini 384- Dussumieria acuta (C.V.) ". 384 Dus8umieria hasselti Blkr. 384 Ehirava jluviatiz.i8 Deraniyagala 385 Stolephorus malabaricu.9 Day 385 Subfamily Clupeini 385 IJarengula, punctata (RUpp.) 385 , Ilarcngula 'l:itteta (C. V .) .. 386 Sardinella albella.<C.V.) 386 Sardinella clupeoides (Blkr.) 387 Sardinella dayi Reg. 387 Saidinella jimbriata (C.V.) .. 387 Sa-rdinella gibbosa (Blkr.) 387 Sarain,ella longiceps C. V. 388 Sardinella melon1#tra (C.) 388 Sard·inella 8inden~i8 (Day) 389 Sardinella sirm (RliPll.) 389 Hilsu U·isha (Ham.) 389 HUsa !'anglt'rta (Blkr.) 390 390 lli/sa tol~ (C.v.) ., . [ 377 ] Q 378 Records of tll.e Indian Museum. [VOL. XLV .P~OE. (}ac1lUsia chapra (Ham.) 391 GadU8ia vari8!Jata (Day) 391 l(owala coval (C.) · . 392 Oarica Bohoma Ham. 392 Ilisha brachllsom:a (Blkr.) .. 3\J2 llisha elongata (Benn.) .. 393 llish.Q jiligera (C. V.) Ii • 393 llislla indica (Swns.) .. 393 ilisha kampeni (Web. & de Bfrt.) 394 llisha leach.enaulti (C.V.) , . -

Molecular Phylogeny and the Evolution of an Adaptive Visual System in Deep-Sea Dragonfishes (Stomiiformes: Stomiidae)
ORIGINAL ARTICLE doi:10.1111/evo.12322 THE COMPLEX EVOLUTIONARY HISTORY OF SEEING RED: MOLECULAR PHYLOGENY AND THE EVOLUTION OF AN ADAPTIVE VISUAL SYSTEM IN DEEP-SEA DRAGONFISHES (STOMIIFORMES: STOMIIDAE) Christopher P. Kenaley,1,2 Shannon C. DeVaney,3 and Taylor T. Fjeran4 1Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachusetts 02138 2E-mail: [email protected] 3Life Science Department, Los Angeles Pierce College, Woodland Hills, California 91371 4College of Forestry, Oregon State University, Corvallis, Oregon 97331 Received December 12, 2012 Accepted November 12, 2013 The vast majority of deep-sea fishes have retinas composed of only rod cells sensitive to only shortwave blue light, approximately 480–490 nm. A group of deep-sea dragonfishes, the loosejaws (family Stomiidae), possesses far-red emitting photophores and rhodopsins sensitive to long-wave emissions greater than 650 nm. In this study, the rhodopsin diversity within the Stomiidae is surveyed based on an analysis of rod opsin-coding sequences from representatives of 23 of the 28 genera. Using phylogenetic inference, fossil-calibrated estimates of divergence times, and a comparative approach scanning the stomiid phylogeny for shared genotypes and substitution histories, we explore the evolution and timing of spectral tuning in the family. Our results challenge both the monophyly of the family Stomiidae and the loosejaws. Despite paraphyly of the loosejaws, we infer for the first time that far-red visual systems have a single evolutionary origin within the family and that this shift in phenotype occurred at approximately 15.4 Ma. In addition, we found strong evidence that at approximately 11.2 Ma the most recent common ancestor of two dragonfish genera reverted to a primitive shortwave visual system during its evolution from a far-red sensitive dragonfish. -
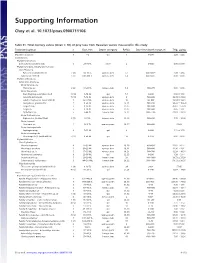
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Análise Dos Padrões De Diversidade Da Fauna Acompanhante Da Pesca De Camarão De Profundidade No Sudeste-Sul Do Brasil
UNIVERSIDADE DO VALE DO ITAJAÍ Centro de Ciências Tecnológicas da Terra e do Mar – CTTMar Curso de Oceanografia Análise dos padrões de diversidade da fauna acompanhante da pesca de camarão de profundidade no Sudeste-Sul do Brasil Débora Alves Pereira Itajaí 2011 UNIVERSIDADE DO VALE DO ITAJAÍ Centro de Ciências Tecnológicas da Terra e do Mar – CTTMar Curso de Oceanografia Análise dos padrões de diversidade da fauna acompanhante da pesca de camarão de profundidade no Sudeste-Sul do Brasil Débora Alves Pereira Trabalho de Conclusão apresentado ao curso de Oceanografia, para a obtenção do grau de Oceanógrafo. Orientador: José Angel Alvarez Perez, PhD Itajaí 2011 Aos meus pais Saulo e Marlene e meus tios Edna e Roberto, o meu maior amor e respeito. i AGRADECIMENTOS Agradeço a Deus por ter me guiado até aqui e me dado força para concluir esta pequena etapa. Obrigado por fazer desta a minha vida. Aos meus pais, Saulo e Marlene, pelas madrugadas de trabalho para me permitir chegar até aqui. Por estarem sempre certos e me educarem para o bem. Por serem firmes e justos. Por me mostrarem o caminho. A eles, meu eterno agradecimento e amor incondicional. A minha irmã Lis, pelos saudáveis momentos de discussões intermináveis entre irmãos, a estranha cumplicidade que só existe entre pessoas que saíram do mesmo ventre. Te amo! A Neném, minha babá, que desde seus nove anos me criou como se fosse sua filha. As minhas avós Maria e Higina, pelo amor, orações, comidas gostosas e por todos os ensinamentos que só quem viveu tantos anos pode mostrar. -
Visual Acuity in Pelagic Fishes and Mollusks
W&M ScholarWorks VIMS Articles 2013 Visual acuity in pelagic fishes and mollusks YL Gagnon TT Sutton S Johnsen Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons Recommended Citation Gagnon, YL; Sutton, TT; and Johnsen, S, "Visual acuity in pelagic fishes and mollusks" (2013). VIMS Articles. 885. https://scholarworks.wm.edu/vimsarticles/885 This Article is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Vision Research 92 (2013) 1–9 Contents lists available at ScienceDirect Vision Research journal homepage: www.elsevier.com/locate/visres Visual acuity in pelagic fishes and mollusks ⇑ Yakir L. Gagnon a, , Tracey T. Sutton b, Sönke Johnsen a a Department of Biology, Duke University, Durham, NC 27708, USA b College of William & Mary, Virginia Institute of Marine Science, Gloucester Point, VA, USA article info abstract Article history: In the sea, visual scenes change dramatically with depth. At shallow and moderate depths (<1000 m), Received 26 June 2013 there is enough light for animals to see the surfaces and shapes of prey, predators, and conspecifics. This Received in revised form 13 August 2013 changes below 1000 m, where no downwelling daylight remains and the only source of light is biolumi- Available online 30 August 2013 nescence. These different visual scenes require different visual adaptations and eye morphologies. In this study we investigate how the optical characteristics of animal lenses correlate with depth and ecology. -

Species Composition, Abundance, and Vertical Distribution of the Stomiid (Pisces: Stomiiformes) Fish Assemblage of the Gulf of Mexico
BULLETIN OF MARINE SCIENCE, 59(3): 530-542, 1996 SPECIES COMPOSITION, ABUNDANCE, AND VERTICAL DISTRIBUTION OF THE STOMIID (PISCES: STOMIIFORMES) FISH ASSEMBLAGE OF THE GULF OF MEXICO Tracey T, Sutton and Thomas L. Hopkins ABSTRACT Species composition, abundance, and vertical distribution of the stomiid fish assemblage were investigated in the eastern Gulf of Mexico, a low-latitude, oligotrophic oceanic ecosys- tem. Seventy-two described species, representing 18 genera, and one undescribed species were identified from 1155 trawl samples. With an additional 10 species reported elsewhere, the stomiid species number now known equals 83, making the Stomiidae the most diverse fish family in the Gulf of Mexico. The assemblage was dominated by three species, Pholos- lomias guemei, Chauliodus s/oani and Stomias affinis. These species, as well as four other common species, exhibited an asynchronous diel vertical migration pattern (450-900 m dur- ing day; 20-300, 550-900 m at night). The percentage of the populations of the three dom- inant species migrating daily ranged from 50-70%. Two other patterns occurred in less abundant species: synchronous migration (400-700 m during the day, 0-200 m at night); and, possible migration from the bathypelagial (> 1000 m during day; 50-300 m at night). Minimum abundance and biomass estimates for the entire assemblage were 1.86 X 105 in- dividuals and 35.3 kg DW·km-2 in the upper 1000 m. Stomiids comprised approximately 10% of the micronekton standing stock in the eastern Gulf. Extrapolating eastern Gulf data to the world warm-water mesopelagial, abundance results suggest that stomiids are the dom- inant mesopelagic upper-trophic level predatory fishes, and as such may serve as key trophic mediators in the transfer of energy in these ecosystems. -

Inventory and Atlas of Corals and Coral Reefs, with Emphasis on Deep-Water Coral Reefs from the U
Inventory and Atlas of Corals and Coral Reefs, with Emphasis on Deep-Water Coral Reefs from the U. S. Caribbean EEZ Jorge R. García Sais SEDAR26-RD-02 FINAL REPORT Inventory and Atlas of Corals and Coral Reefs, with Emphasis on Deep-Water Coral Reefs from the U. S. Caribbean EEZ Submitted to the: Caribbean Fishery Management Council San Juan, Puerto Rico By: Dr. Jorge R. García Sais dba Reef Surveys P. O. Box 3015;Lajas, P. R. 00667 [email protected] December, 2005 i Table of Contents Page I. Executive Summary 1 II. Introduction 4 III. Study Objectives 7 IV. Methods 8 A. Recuperation of Historical Data 8 B. Atlas map of deep reefs of PR and the USVI 11 C. Field Study at Isla Desecheo, PR 12 1. Sessile-Benthic Communities 12 2. Fishes and Motile Megabenthic Invertebrates 13 3. Statistical Analyses 15 V. Results and Discussion 15 A. Literature Review 15 1. Historical Overview 15 2. Recent Investigations 22 B. Geographical Distribution and Physical Characteristics 36 of Deep Reef Systems of Puerto Rico and the U. S. Virgin Islands C. Taxonomic Characterization of Sessile-Benthic 49 Communities Associated With Deep Sea Habitats of Puerto Rico and the U. S. Virgin Islands 1. Benthic Algae 49 2. Sponges (Phylum Porifera) 53 3. Corals (Phylum Cnidaria: Scleractinia 57 and Antipatharia) 4. Gorgonians (Sub-Class Octocorallia 65 D. Taxonomic Characterization of Sessile-Benthic Communities 68 Associated with Deep Sea Habitats of Puerto Rico and the U. S. Virgin Islands 1. Echinoderms 68 2. Decapod Crustaceans 72 3. Mollusks 78 E. -

The Exceptional Diversity of Visual Adaptations in Deep-Sea Teleost Fishes
Seminars in Cell and Developmental Biology xxx (xxxx) xxx–xxx Contents lists available at ScienceDirect Seminars in Cell & Developmental Biology journal homepage: www.elsevier.com/locate/semcdb Review The exceptional diversity of visual adaptations in deep-sea teleost fishes Fanny de Busserolles*, Lily Fogg, Fabio Cortesi, Justin Marshall Queensland Brain Institute, The University of Queensland, St Lucia, Queensland 4072, Australia ARTICLE INFO ABSTRACT Keywords: The deep-sea is the largest and one of the dimmest habitats on earth. In this extreme environment, every photon Deep-sea teleost counts and may make the difference between life and death for its inhabitants. Two sources of light are present Dim-light vision in the deep-sea; downwelling light, that becomes dimmer and spectrally narrower with increasing depth until Ocular adaptation completely disappearing at around 1000 m, and bioluminescence, the light emitted by animals themselves. Retina Despite these relatively dark and inhospitable conditions, many teleost fish have made the deep-sea their home, Opsin relying heavily on vision to survive. Their visual systems have had to adapt, sometimes in astonishing and Bioluminescence bizarre ways. This review examines some aspects of the visual system of deep-sea teleosts and highlights the exceptional diversity in both optical and retinal specialisations. We also reveal how widespread several of these adaptations are across the deep-sea teleost phylogeny. Finally, the significance of some recent findings as well as the surprising diversity in visual adaptations is discussed. 1. Introduction or mate detection, to communicate, camouflage, or for navigation, in- cluding to stay within a particular depth range [4,5]. -

And Bathypelagic Fish Interactions with Seamounts and Mid-Ocean Ridges
Meso- and bathypelagic fish interactions with seamounts and mid-ocean ridges Tracey T. Sutton1, Filipe M. Porteiro2, John K. Horne3 and Cairistiona I. H. Anderson3 1 Harbor Branch Oceanographic Institution, 5600 US Hwy. 1 N, Fort Pierce FL, 34946, USA (Current address: Virginia Institute of Marine Science, P.O.Box 1346, Gloucester Point, Virginia 23062-1346) 2 DOP, University of the Azores, Horta, Faial, the Azores 3 School of Aquatic and Fishery Sciences, University of Washington, Seattle WA, 98195, USA Contact e-mail (Sutton): [email protected]"[email protected] Tracey T. Sutton T. Tracey Abstract The World Ocean's midwaters contain the vast majority of Earth's vertebrates in the form of meso- and bathypelagic ('deep-pelagic,' in the combined sense) fishes. Understanding the ecology and variability of deep-pelagic ecosystems has increased substantially in the past few decades due to advances in sampling/observation technology. Researchers have discovered that the deep sea hosts a complex assemblage of organisms adapted to a “harsh” environment by terrestrial standards (i.e., dark, cold, high pressure). We have learned that despite the lack of physical barriers, the deep-sea realm is not a homogeneous ecosystem, but is spatially and temporally variable on multiple scales. While there is a well-documented reduction of biomass as a function of depth (and thus distance from the sun, ergo primary production) in the open ocean, recent surveys have shown that pelagic fish abundance and biomass can 'peak' deep in the water column in association with abrupt topographic features such as seamounts and mid-ocean ridges. We review the current knowledge on deep-pelagic fish interactions with these features, as well as effects of these interactions on ecosystem functioning. -

Phylogenetic Interrelationships of the Stomiid Fishes (Teleostei: Stomiiformes)
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, THE UNIVERSITY OF MICHIGAN NO. 171 Phylogenetic Interrelationships of the Stomiid Fishes (Teleostei: Stomiiformes) by William L. Fink Division of Biological Sciences and Museum of Zoology The University of Michigan Ann Arbor, Michigan 48 109-1079 Ann Arbor MUSEUM OF ZOOLOGY, THE UNIVERSITY OF MICHIGAN December 3 1, 1985 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN NO. 171 The publications of the Museum of Zoology, The University of Michigan, consist of two series-the Occasional Papers and the Miscellaneous Publications. Both series were founded by Dr. Bryant Walker, Mr. Bradshaw H. Swales, and Dr. W. W. Newcomb. The Occasional Papers, initiated in 1913, serve as a medium for original studies based principally upon the collections in the Museum. They are issued separately. When a sufficient number of pages has been printed to form a volume, the Museum will supply a title page, table of contents, and an index to libraries and individuals on the mailing list for the series. The Miscellaneous Publications, which include papers on field and museum techniques, monographic studies, and other contributions not within the scope of the Occasional Papers, were established in 1916 and are published separately. It is not intended that they be grouped into volumes. Each number has a title page and, when necessary, a table of contents. A complete list of publications on Birds, Fishes, Insects, Mammals, Mollusks, and Reptiles and Amphibians is available. Address inquiries to the Director, Museum of Zoology, Ann Arbor, Michigan 48109-1079. MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOI,OGY, THE UNIVERSITY OF MICHIGAN NO.