Technological Gaps Hindering Uptake of Crms Substitution in Industrial Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hfc STRUCTURAL FOAMS SYNTHESIZED from POLYMER PRECURSORS
HfC STRUCTURAL FOAMS SYNTHESIZED FROM POLYMER PRECURSORS Except where reference is made to the work of others, the work described in this dissertation is my own or was done in collaboration with my advisory committee. This dissertation does not include proprietary or classified information. ________________________________________ Haibo Fan Certificate of Approval: ______________________________ _____________________________ ZhongYang Cheng Bryan A. Chin, Chair Assistant Professor Professor Materials Engineering Materials Engineering ______________________________ ______________________________ Dong-Joo Kim William C. Neely Assistant Professor Professor Materials Engineering Chemistry and Biochemistry ___________________ Stephen L. McFarland Dean Graduate School HfC STRUCTURAL FOAMS SYNTHESIZED FROM POLYMER PRECURSORS Haibo Fan A Dissertation Submitted to the Graduate Faculty of Auburn University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Auburn, Alabama December 16, 2005 HfC STRUCTURAL FOAMS SYNTHESIZED FROM POLYMER PRECURSORS Haibo Fan Permission is granted to Auburn University to make copies of this dissertation at its discretion, upon request of individuals or institutions and at their expense. The author reserves all publication rights. ______________________________ Signature of Author ______________________________ Date iii VITA Haibo Fan, son of Chaoying Fan and Yulan Shi, was born on November 18, 1976, in Ugrat Front Banner, Inner-Mongolia, the People’s Republic of China. He graduated from Ugrat Front Banner No.1 High School in 1994. He studied at Tianjin University for four years and graduated with a Bachelor of Engineering degree in Mechanical Engineering in July 1998. He entered Auburn University in August 2000 to pursue his M.S. and Ph.D. degrees in Materials Engineering. He received his M.S. degree in May 2003. -
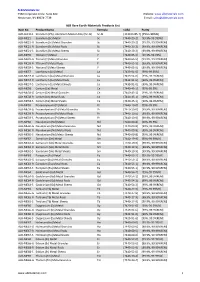
ALB Materials Inc Product List.Xlsx
ALB Materials Inc 2360 Corporate Circle. Suite 400 Website: www.albmaterials.com Henderson, NV 89074-7739 E-mail: [email protected] ALB Rare Earth Materials Products List Item No. Product Name Formula CAS# Purity ALB-AL2113 Scandium (2%)- Aluminum Master Alloy (Sc-Al) Sc-Al [113413-85-7] [2%Sc+98%Al] ALB-ME21 Scandium (Sc) Metal Sc [7440-20-2] [99.9%-99.999%] ALB-ME21-G Scandium (Sc) Metal Granules Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME21-R Scandium (Sc) Metal Rods Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME21-S Scandium (Sc) Metal Sheets Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME39 Yttrium (Y) Metal Y [7440-65-5] [99.9%-99.99%] ALB-ME39-G Yttrium (Y) Metal Granules Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME39-R Yttrium (Y) Metal Rods Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME39-S Yttrium (Y) Metal Sheets Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME57 Lanthanum (La) Metal La [7439-91-0] [99%-99.95%] ALB-ME57-G Lanthanum (La) Metal Granules La [7439-91-0] [99%, 99.9%REM] ALB-ME57-R Lanthanum (La) Metal Rods La [7439-91-0] [99%, 99.9%REM] ALB-ME57-S Lanthanum (La) Metal Sheets La [7439-91-0] [99%, 99.9%REM] ALB-ME58 Cerium (Ce) Metal Ce [7440-45-1] [99%-99.9%] ALB-ME58-G Cerium (Ce) Metal Granules Ce [7440-45-1] [99%, 99.9%REM] ALB-ME58-R Cerium (Ce) Metal Rods Ce [7440-45-1] [99%, 99.9%REM] ALB-ME58-S Cerium (Ce) Metal Sheets Ce [7440-45-1] [99%, 99.9%REM] ALB-ME59 Praseodymium (Pr) Metal Pr [7440-10-0] [99%-99.9%] ALB-ME59-G Praseodymium (Pr) Metal Granules Pr [7440-10-0] [99.9%, 99.99%REM] ALB-ME59-R Praseodymium (Pr) Metal Rods Pr [7440-10-0] -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

(12) United States Patent (10) Patent No.: US 9,048,183 B2 Ganguli Et Al
USOO9048183B2 (12) United States Patent (10) Patent No.: US 9,048,183 B2 Ganguli et al. (45) Date of Patent: Jun. 2, 2015 (54) NMOSMETAL GATE MATERIALS, (52) U.S. Cl. MANUFACTURING METHODS, AND CPC .......... HOIL 21/28008 (2013.01); C23C I6/06 EQUIPMENT USING CVD AND ALD (2013.01); C23C 16/32 (2013.01); PROCESSES WITH METAL BASED (Continued) PRECURSORS (58) Field of Classification Search (71) Applicant: Applied Materials, Inc., Santa Clara, None CA (US) See application file for complete search history. (56) References Cited (72) Inventors: Seshadri Ganguli, Sunnyvale, CA (US); Srinivas Gandikota, Santa Clara, CA U.S. PATENT DOCUMENTS (US); Yu Lei, Belmont, CA (US); Xinliang Lu, Fremont, CA (US); Sang 5,055,280 A 10/1991 Nakatani et al. Ho Yu, Cupertino, CA (US); Hoon Kim, 6,139,922 A 10/2000 Kaloyeros et al. Santa Clara, CA (US); Paul F. Ma, Santa (Continued) Clara, CA (US); Mei Chang, Saratoga, CA (US); Maitreyee Mahajani, FOREIGN PATENT DOCUMENTS Saratoga, CA (US); Patricia M. Liu, Saratoga, CA (US) KR 2003OOO9093. A 1, 2003 s OTHER PUBLICATIONS (73) Assignee: APPLIED MATERIALS, INC., Santa International Search Report PCT/US2011/033820 dated Jan. 5, Clara, CA (US) 2012. (*) Notice: Subject to any disclaimer, the term of this (Continued) patent is extended or adjusted under 35 Primary Examiner — Yasser A Abdelaziez U.S.C. 154(b) by 0 days. (74) Attorney, Agent, or Firm — Patterson & Sheridan, LLP (21) Appl. No.: 14/147,291 (57) ABSTRACT Embodiments provide methods for depositing metal-contain (22) Filed: Jan. 3, 2014 ing materials. The methods include deposition processes that form metal, metal carbide, metal silicide, metal nitride, and (65) Prior Publication Data metal carbide derivatives by a vapor deposition process, including thermal decomposition, CVD, pulsed-CVD, or US 2014/O12O712 A1 May 1, 2014 ALD. -

High-Temperature Mechanical Properties of Polycrystalline Hafnium Carbide and Hafnium Carbide Containing 13-Volume-Percent Hafnium Diboride Nasa Tn D-5008
d HIGH-TEMPERATURE MECHANICAL PROPERTIES OF POLYCRYSTALLINE HAFNIUM CARBIDE AND HAFNIUM CARBIDE CONTAINING 13-VOLUME-PERCENT HAFNIUM DIBORIDE NASA TN D-5008 HIGH-TEMPERATURE MECHANICAL PROPERTIES OF POLYCRYSTALLINE HAFNIUM CARBIDE AND HAFNIUM CARBIDE CONTAINING 13-VOLUME- PERCENT HAFNIUM DIBORIDE By William A. Sanders and Hubert B. Probst Lewis Research Center Cleveland, Ohio NATIONAL AERONAUTICS AND SPACE ADMINISTRATION ~ For sale by the Clearinghouse for Federal Scientific and Technical Information Springfield, Virginia 22151 - CFSTl price $3.00 ABSTRACT Hot-pressed, single-phase HfC and HfC containing 13-vol % HfB2 were tested in three-point transverse rupture to temperatures as high as 4755' F (2625' C). Hot hard- ness tests were also run to 3200' F (1760' C). Separate effects on the transverse rup- ture behavior of HfC due to the HfB2 second phase and due to a grain- size difference are discussed on the basis of strength, deformation, and metallographic results. The probable mechanisms responsible for deformation are also discussed. In hot-hardness tests of HfC containing 13 vol % HfB2 second phase, a change in the temperature de- pendency of hot hardness at 2600' F (1425' C) is discussed and related to the degree of cracking around indentations. ii H I G H -TEM PERATURE MEC HANICAL PRO PE RTlES OF POLY C RY STALLINE HAFNIUM CARBIDE AND HAFNIUM CARBIDE CONTAINING 13 -VOLUME -PERCENT HAFN IUM D1 BOR I DE by William A. Sanders and Hubert 9. Probst Lewis Research Center SUMMARY Transverse rupture tests were conducted on single -phase hafnium carbide and hafnium carbide containing 13- volume- percent hafnium diboride as a distinct second phase. -

Oxidation Mechanisms of Hafnium Carbide and Hafnium Diboride in the Temperature Range 1400 to 2100°C
C. BRENT BARGERON, RICHARD C. BENSON, ROBB W. NEWMAN, A. NORMAN JETTE, and TERRY E. PHILLIPS OXIDATION MECHANISMS OF HAFNIUM CARBIDE AND HAFNIUM DIBORIDE IN THE TEMPERATURE RANGE 1400 TO 2100°C Two ultra-high-temperature materials, hafnium carbide and hafnium diboride, were oxidized in the temperature range 1400 to 2100°C. The two materials oxidized in distinctly different ways. The carbide formed a three-layer system consisting of a layer of residual carbide, a layer of reduced (partially oxidized) hafnium oxide containing carbon, and a layer of fully oxidized hafnium dioxide. The diboride oxidized into only two layers. For the diboride system, the outer layer, mainly hafnium dioxide, contained several intriguing physical structures. INTRODUCTION Materials that can provide protection at temperatures aspects of research that has been performed over the past above l700°C in an oxidative environment are needed for four or five years.2-5 important applications. To be usefully employed as a turbine blade coating, for example, a substance would EXPERIMENTAL METHODS need to withstand many excursions from normal ambient The experimental arrangement for the oxidation pro conditions into the high-temperature regime and back cess has been described in detail previously.2-4 An induc again without cracking, spalling, or ablating. Other ap tion furnace consisting of two concentric zirconia tubes plications, such as a combustion chamber liner, might with a graphite susceptor between them was used to heat require only one high-temperature exposure. Not only do the specimen. (A susceptor is the heating element in an the chemical properties need to be considered, but the induction furnace.) The specimen temperature was mea physical, microscopic structure of a candidate material sured with an optical pyrometer through a sapphire win can also determine how well it will function under ex dow. -

Carbides and Nitrides of Zirconium and Hafnium
materials Review Carbides and Nitrides of Zirconium and Hafnium Sergey V. Ushakov 1,* , Alexandra Navrotsky 1,* , Qi-Jun Hong 2,* and Axel van de Walle 2,* 1 Peter A. Rock Thermochemistry Laboratory and NEAT ORU, University of California at Davis, Davis, CA 95616, USA 2 School of Engineering, Brown University, Providence, RI 02912, USA * Correspondence: [email protected] (S.V.U.); [email protected] (A.N.); [email protected] (Q.-J.H.); [email protected] (A.v.d.W.) Received: 6 August 2019; Accepted: 22 August 2019; Published: 26 August 2019 Abstract: Among transition metal carbides and nitrides, zirconium, and hafnium compounds are the most stable and have the highest melting temperatures. Here we review published data on phases and phase equilibria in Hf-Zr-C-N-O system, from experiment and ab initio computations with focus on rocksalt Zr and Hf carbides and nitrides, their solid solutions and oxygen solubility limits. The systematic experimental studies on phase equilibria and thermodynamics were performed mainly 40–60 years ago, mostly for binary systems of Zr and Hf with C and N. Since then, synthesis of several oxynitrides was reported in the fluorite-derivative type of structures, of orthorhombic and cubic higher nitrides Zr3N4 and Hf3N4. An ever-increasing stream of data is provided by ab initio computations, and one of the testable predictions is that the rocksalt HfC0.75N0.22 phase would have the highest known melting temperature. Experimental data on melting temperatures of hafnium carbonitrides are absent, but minimum in heat capacity and maximum in hardness were reported for Hf(C,N) solid solutions. -

Crystal Structure Transformations in Binary Halides
1 A UNITED STATES DEPARTMENT OF A111D3 074^50 IMMERCE JBLICAT10N NSRDS—NBS 41 HT°r /V\t Co^ NSRDS r #C£ DM* ' Crystal Structure Transformations in Binary Halides u.s. ARTMENT OF COMMERCE National Bureau of -QC*-| 100 US73 ho . 4 1^ 72. NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation’s science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation’s physical measure- ment system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scien- tific community, industry, and commerce. The Institute consists of a Center for Radia- tion Research, an Office of Measurement Services and the following divisions: Applied Mathematics—Electricity—Heat—Mechanics—Optical Physics—Linac Radiation 2—Nuclear Radiation 2—Applied Radiation 2—Quantum Electronics 3— Electromagnetics 3—Time and Frequency 3—Laboratory Astrophysics 3—Cryo- 3 genics . -

A Study of the Mechanical Properties of Silicon-Based Thin Films
MECHANICAL PROPERTIES OF SILICON-BASED FILMS FABRICATED BY PECVD A STUDY OF THE MECHANICAL PROPERTIES OF SILICON-BASED THIN FILMS DEPOSITED BY ECR-PECVD AND ICP-CVD By OWEN TAGGART, B.ENG. A Thesis Submitted to the School of Graduate Studies in Partial Fulfillment of the Requirements for the Degree Master of Applied Science McMaster University © Copyright by Owen Taggart, April 2013 McMaster University MASTER OF APPLIED SCIENCE (2013) Hamilton, Ontario (Engineering Physics) Title: A Study of the Mechanical Properties of Silicon-Based Thin Films Deposited by ECR-PECVD and ICP-CVD AUTHOR: Owen Taggart SUPERVISOR: Professor Peter Mascher Number of pages: xv, 151 ii Abstract Silicon-based dielectric thin films including amorphous hydrogenated aluminium-doped silicon oxides (a-SiAlxOy:H), amorphous hydrogenated silicon nitrides (a-SiNx:H), and amorphous hydrogenated silicon carbides (a-SiCx:H) were deposited by remote plasma chemical vapour deposition (RPECVD) techniques including electron cyclotron resonance plasma enhanced chemical vapour deposition (ECR-PECVD) and inductively-coupled-plasma chemical vapour deposition (ICP-CVD) on silicon (Si) wafers, soda-lime glass microscope slides, and glassy carbon (C) plates. Aluminium (Al) in the SiAlO films was incorporated by way of a metalorganic Al(TMHD)3 precursor. Thickness, refractive index, and growth rate of the films were measured using variable angle spectroscopic ellipsometry (VASE). Film composition was measured using energy dispersive X-ray spectroscopy (EDX) for the SiAlO films and Rutherford backscattering spectrometry (RBS) for the SiCx films. Elastic modulus and hardness of the SiAlO and SiCx films were measured using nanoindentation and their adhesion was characterized via progressive load scratch testing. -

Quantification of Hafnium in Selected Inorganic and Organometallic Compounds
Quantification of hafnium in selected inorganic and organometallic compounds A dissertation submitted to meet the requirements for the degree of Magister Scientiae in the FACULTY OF NATURAL AND AGRICULTURAL SCIENCES DEPARTMENT OF CHEMISTRY at the UNIVERSITY OF THE FREE STATE BLOEMFONTEIN by GONTSE ATLHOLANG ADELINE MALEFO Supervisor Prof. W. Purcell Co-supervisors Dr. J.T. Nel and Dr. M Nete February 2016 Declaration I declare that the thesis entitled “THE QUANTIFICATION OF HAFNIUM IN SELECTED INORGANIC AND ORGANOMETALLIC COMPOUNDS” submitted for the degree Magister in Analytical Chemistry, at the University of the Free State is my own original work and has not been previously submitted to any other institution of higher education in the Republic of South Africa or abroad. I further declare that all sources cited or quoted are indicated and acknowledged by means of a comprehensive list of references. Signature………………………………… Date…………………..…………. Gontse Atlholang Adeline Malefo Acknowledgements I would like to thank and express my sincere gratitude to; My Lord and saviour, Jesus Christ for his grace, divine guidance and all the strength bestowed upon me throughout the research project. My supervisor, Prof. W. Purcell for his guidance and patient support throughout the research project. His expertise and understanding contributed greatly to my knowledge in the field of study. My co-supervisor, Dr. Nel, for his valuable contributions in assisting with reviews of each chapter which greatly improved/enhanced the content my thesis. My co-supervisor, Dr. M. Nete, for his continuous encouragement and support. He was very helpful to the completion of this study. Analytical chemistry group (Deidre, Dika, Hlengiwe, Enerstine, Melaku, Ntoi, Sibongile, Sumit, Trevor and Qinisile) for providing a conducive work environment. -

Solvo-Hydrothermal Growth and Photoluminescence Studies of Micro and Nano Structured Zinc Sulfide for Bio-Imaging Applications
Solvo-hydrothermal growth and photoluminescence studies of micro and nano structured Zinc Sulfide for bio-imaging applications Thesis submitted to COCHIN UNIVERSITY OF SCIENCE AND TECHNOLOGY in partial fulfillment of the requirements for the award of the degree of DOCTOR OF PHILOSOPHY in Physics by Sajan P. Nano Functional Materials Lab Department of Physics Cochin University of Science and Technology Cochin - 682 022, Kerala, India June 2016 Solvo-hydrothermal growth and photoluminescence studies of micro and nano structured Zinc Sulfide for bio-imaging applications Ph.D. thesis in the field of Physics Author: Sajan P. Nano Functional Materials Lab Department of Physics Cochin University of Science and Technology Cochin - 682 022, Kerala, India. Email: [email protected] Supervisor: Dr. M. Junaid Bushiri Professor Nano Functional Materials Lab Department of Physics Cochin University of Science and Technology Cochin-682 022, Kerala, India. Email: [email protected] June 2016 Dedicated to my mother Department of Physics Cochin University of Science and Technology Cochin – 682 022 Dr. M. Junaid Bushiri Professor Department of Physics Cochin University of Science and Technology Cochin 682 022, India. Certified that the work presented in this thesis entitled “Solvo-hydrothermal growth and photoluminescence studies of micro and nano structured Zinc Sulfide for bio- imaging applications” is based on the bonafide record of research work done by Sajan P., under my guidance in the Department of Physics, Cochin University of Science and Technology, Cochin 682 022 in partial fulfillment of the requirements for the award of degree of Doctor of Philosophy and has not been included in any other thesis submitted for the award of any degree. -

Appendix a Further Reading
Appendix A Further Reading D.M. Adams, Inorganic Solids, Wiley, New York, 1974. Very good older book with excellent figures. It emphasizes close packing. L.V. Azaroff, Introduction to Solids, McGraw-Hill, New York, 1960. L. Bragg, The Crystalline State, G. Bell and Sons, London, 1965. L. Bragg and G.F. Claringbull, Crystal Structures of Minerals, G. Bell, London, 1965. P.J. Brown and J.B. Forsyth. The Crystal Structure of Solids, E. Arnold, London, 1973. M.J. Buerger, Elementary Crystallography, Wiley, New York, 1956. J.K. Burdett, Chemical Bonding in Solids, Oxford University Press, Oxford, 1992. Cambridge Structural Data Base (CSD). Cambridge Crystallographic Data Centre, University Chemical Laboratory, Cambridge, England. C.R.A. Catlow, Ed., Computer Modelling in Inorganic Crystallography, Academic Press, San Diego, 1997. A.K. Cheetham and P. Day, Solid-State Chemistry, Techniques, Clarendon, Ox- ford, 1987. P.A. Cox, Transition Metal Oxides, Oxford University Press, Oxford, 1992. CrystalMaker, A powerful computer program for the Macintosh and Windows by David Palmer, CrystalMaker Software Ltd., Yarnton, Oxfordshire, UK. This program was used for many figures and it aided greatly in interpreting many structures for this book and accompanying CD. B.D. Cullity, Elements of X-ray Diffraction, Addison-Wesley, Reading, MA, 1956. J. Donohue, The Structure of The Elements, Wiley, New York, 1974. The most comprehensive coverage of the structures of elements. B.E. Douglas, D.H. McDaniel, and J.J. Alexander, Concepts and Models of Inor- ganic Chemistry, 3rd ed., Wiley, New York, 1994. The PTOT system is discussed and applied briefly. F.S. Galasso, Structure, Properties and Preparation of Perovskite-Type Compounds, Pergamon, Oxford, 1969.