Advancing the Ferret As an Immunological Model to Study B-Cell Responses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

June 18 Newsletter
ESSEX EGYPTOLOGY GROUP Newsletter 114 June/July 2018 DATES FOR YOUR DIARY 3rd June The Tomb of Tatia at Saqqara: Vincent Oeters 1st July Papyrus Berlin P10480-82: a Middle Kingdom mortuary ritual reflected in writing: Dr Ilona Regulski 5th August Flies, lions and oysters: military awards or tea for two: Taneash Sidpura Annual General Meeting 2nd September Egypt’s Origins: the view from Mesopotamia and Iran: Dr Paul Collins This month we welcome back Vincent Oeters from Holland. During the 2009 field season of the joint mission of the Rijksmuseum van Oudheden (National Museum of Antiquities) at Leiden, the Netherlands and Leiden University (Faculty of Humanities, Department of Egyptology) a small Ramesside tomb-chapel was unearthed in the New Kingdom necropolis at Saqqara (1550-1070 BC), south of the causeway of Unas. The tomb-chapel belonged to a man named Tatia, Priest of the front of Ptah and Chief of the Goldsmiths. By studying the reliefs as well as the architecture and by comparing the tomb with other Ramesside tombs at Saqqara and elsewhere, an attempt was made to establish a more precise dating of the monument. Recent research has resulted in new insights on Tatia and his career, signs of private devotion and familial relationships. It appears Tatia was a relative of two other well-known New Kingdom officials, one whose important tomb was also built at Saqqara, the other a vizier and 'High Priest of Amun' in Thebes. In July we welcome, Ilona Regulski, the curator responsible for the papyrus collection and other inscribed material in the collection at the British Museum. -

Needle Roller and Cage Assemblies B-003〜022
*保持器付針状/B001-005_*保持器付針状/B001-005 11/05/24 20:31 ページ 1 Needle roller and cage assemblies B-003〜022 Needle roller and cage assemblies for connecting rod bearings B-023〜030 Drawn cup needle roller bearings B-031〜054 Machined-ring needle roller bearings B-055〜102 Needle Roller Bearings Machined-ring needle roller bearings, B-103〜120 BEARING TABLES separable Self-aligning needle roller bearings B-121〜126 Inner rings B-127〜144 Clearance-adjustable needle roller bearings B-145〜150 Complex bearings B-151〜172 Cam followers B-173〜217 Roller followers B-218〜240 Thrust roller bearings B-241〜260 Components Needle rollers / Snap rings / Seals B-261〜274 Linear bearings B-275〜294 One-way clutches B-295〜299 Bottom roller bearings for textile machinery Tension pulleys for textile machinery B-300〜308 *保持器付針状/B001-005_*保持器付針状/B001-005 11/05/24 20:31 ページ 2 B-2 *保持器付針状/B001-005_*保持器付針状/B001-005 11/05/24 20:31 ページ 3 Needle Roller and Cage Assemblies *保持器付針状/B001-005_*保持器付針状/B001-005 11/05/24 20:31 ページ 4 Needle roller and cage assemblies NTN Needle Roller and Cage Assemblies This needle roller and cage assembly is one of the or a housing as the direct raceway surface, without using basic components for the needle roller bearing of a inner ring and outer ring. construction wherein the needle rollers are fitted with a The needle rollers are guided by the cage more cage so as not to separate from each other. The use of precisely than the full complement roller type, hence this roller and cage assembly enables to design a enabling high speed running of bearing. -

Emission Station List by County for the Web
Emission Station List By County for the Web Run Date: June 20, 2018 Run Time: 7:24:12 AM Type of test performed OIS County Station Status Station Name Station Address Phone Number Number OBD Tailpipe Visual Dynamometer ADAMS Active 194 Imports Inc B067 680 HANOVER PIKE , LITTLESTOWN PA 17340 717-359-7752 X ADAMS Active Bankerts Auto Service L311 3001 HANOVER PIKE , HANOVER PA 17331 717-632-8464 X ADAMS Active Bankert'S Garage DB27 168 FERN DRIVE , NEW OXFORD PA 17350 717-624-0420 X ADAMS Active Bell'S Auto Repair Llc DN71 2825 CARLISLE PIKE , NEW OXFORD PA 17350 717-624-4752 X ADAMS Active Biglerville Tire & Auto 5260 301 E YORK ST , BIGLERVILLE PA 17307 -- ADAMS Active Chohany Auto Repr. Sales & Svc EJ73 2782 CARLISLE PIKE , NEW OXFORD PA 17350 717-479-5589 X 1489 CRANBERRY RD. , YORK SPRINGS PA ADAMS Active Clines Auto Worx Llc EQ02 717-321-4929 X 17372 611 MAIN STREET REAR , MCSHERRYSTOWN ADAMS Active Dodd'S Garage K149 717-637-1072 X PA 17344 ADAMS Active Gene Latta Ford Inc A809 1565 CARLISLE PIKE , HANOVER PA 17331 717-633-1999 X ADAMS Active Greg'S Auto And Truck Repair X994 1935 E BERLIN ROAD , NEW OXFORD PA 17350 717-624-2926 X ADAMS Active Hanover Nissan EG08 75 W EISENHOWER DR , HANOVER PA 17331 717-637-1121 X ADAMS Active Hanover Toyota X536 RT 94-1830 CARLISLE PK , HANOVER PA 17331 717-633-1818 X ADAMS Active Lawrence Motors Inc N318 1726 CARLISLE PIKE , HANOVER PA 17331 717-637-6664 X 630 HOOVER SCHOOL RD , EAST BERLIN PA ADAMS Active Leas Garage 6722 717-259-0311 X 17316-9571 586 W KING STREET , ABBOTTSTOWN PA ADAMS Active -

Water Quality Assessment Report for United Keno Hill Mines
WATER QUALITY ASSESSMENT REPORT FOR UNITED KENO HILL MINES Report Prepared for: Elsa Reclamation and Development Company Whitehorse, Yukon Report Prepared by: Minnow Environmental Inc. 2 Lamb Street Georgetown, Ontario L7G 3M9 July 2008 WATER QUALITY ASSESSMENT REPORT FOR UNITED KENO HILL MINES Report Prepared for: Elsa Reclamation and Development Company Whitehorse, Yukon Report Prepared by: Minnow Environmental Inc. Cynthia Russel, B.Sc. Project Manager Patti Orr, M.Sc. Technical Reviewer July 2008 ERDC Water Quality Assessment EXECUTIVE SUMMARY United Keno Hill Mines Limited and UKH Minerals Ltd. were the previous owners of the properties located on and around Galena Hill, Keno Hill, and Sourdough Hill collectively known as the United Keno Hill Mining Property (UKHM). The UKHM is located in north- central Yukon Territory and is comprised of approximately 827 mineral claims covering the three mountains (“hills” named above) over an area of approximately 15,000 ha (about 29 km long and 8 km wide). Associated with the site are abandoned adits, buildings/structures, and waste material which represent a source of contaminants to the downstream watersheds. In June 2005, Alexco Resource Corp was selected as the preferred purchaser of the UKHM assets. Alexco’s subsidiary Elsa Reclamation and Development Company (ERDC) is required to develop a Reclamation Plan for the Existing State of the Mine. As part of the closure planning process, long-term water quality performance will need to be assessed relative to relevant water uses and closure plan options. It is expected that historical sources associated with the UKHM may not allow for generic water quality guidelines to be achieved at all downstream locations and that alternative targets may need to be developed, depending on water use goals. -

DS Forrsrejse 2007
DÆS forårsrejse 2007 - steder og personer Fredag 16.3 Dendera tempel: bl.a. stenblokke ved siden af fra MR mm., krypt og tag med zodiac (kopi, original på Louvre) Abydos: Seti Is tempel, Osireion bag Setis tempel og Ramses IIs tempel. Gode billeder på bla.: http://www.egyptarchive.co.uk/html/seti_abydos_index.html og http://www.egyptarchive.co.uk/html/ramesses_abydos_index.html Lørdag 17.3 Theben Vestbredden: Kongernes Dal: det nye Visitor Center med den japanske model af Kongernes Dal, Merneptahs grav KV8 og forskellige grave, bl.a. Tutmosis III KV33, Tausret/Sethnakht KV14, Seti II KV15, Siptah KV47, Ramses III KV11. Gåtur over bjergene fra Kongernes Dal til Deir el Medina, hvor nogle gik via arbejderhytterne. Glem ikke: http://www.kv5.com/ med alt om Kongernes Dal. Deir el Medina: Sennedjems grav TT1 og TT359 Inherkau - nogle så også/eller i stedet TT359 Pashedus grav TT3. Vi var rundt om selve arbejderbyen til det ptolemæiske Hathortempel, de mindre kapeller og den såkaldte ’Great Pit’, der var forsøg på at grave en kæmpe brønd. Der er fantastiske billeder og planer mm. af gravene på: http://www.osirisnet.net/tombes/artisans/e_artis1.htm Pause overfor Medinet Habu (Jørgen og Elsebeth forsøgte at finde et lille tempel i baghaven – jeg tror det må være Qasr el Aguz, som er et lille tempel bygget af Ptolemæus VII for Thoth (PMII, 527-530), se: http://www.egyptsites.co.uk/upper/luxorwest/temples/cult.html . - Tinne og Preben nåede lige et lynvisit i Medinet Habu!). Rekhmires grav TT100 og Sennefers grav TT96. Se også: http://www.osirisnet.net/tombes/nobles/e_nob1.htm med disse grave og mange flere! Søndag 18.3 Formiddag ’fri’, nogle i Karnak eller ved den nyligt frilagte Sfinxallé mm. -

On the Modeling of Air Flow in the Tombs of the Valley of Kings
cs: O ani pe ch n e A c M c Khalil, Fluid Mech Open Acc 2017, 4:3 d e i s u s l F Fluid Mechanics: Open Access DOI: 10.4172/2476-2296.1000166 ISSN: 2476-2296 Research Article Open Access On the Modeling of Air Flow in the Tombs of the Valley of Kings Essam E Khalil1,2* 1Chairman Arab HVAC Code Committee ASHRAE Director-At-Large, USA, Convenor ISO TC205 WG2, Co-Convenor ISO TC163 WG4, Deputy Director (International) AIAA, USA 2DIC, Professor of Mechanical Engineering, Cairo University, Cairo, Egypt Abstract The tombs of the kings in Valley of the Kings, Luxor, are considered to be one of the tourism industry’s bases in Egypt due to their uniqueness all over the world. Hence, they should be preserved from the different factors that might cause harm for their wall paintings. One of these factors is the excessive relative humidity as it increases the bacteria and fungus activity inside the tomb in addition to its effect on the mechanical and physical properties of materials. This chapter describes the Research work to design ventilation systems to some of these important tombs. The chapter aims to investigate, design, and implement controlled climate to the tombs of the valley of kings with complete monitoring of air properties, temperature, relative humidity and carbon oxides and air quality parameters mechanical distributions inside selected tombs of the valley of the kings that are open for visitors. A complete climate control and monitoring of air will be effected with the aid of a mechanical ventilation system extracting air at designated locations in the wooden raised floor of the tombs. -

Il Cristianesimo in Egitto Luci E Ombre in Abydos La Tomba
egittologia.net magazine in questo numero: IL CRISTIANESIMO IN EGITTO EGITTO A VENEZIA LUCI E OMBRE IN ABYDOS SPECIALE NEFERTARI LA TOMBA QV66 AREA ARCHEOLOGICA TEBANA IL VILLAGGIO DI DEIR EL-MEDINA EGITTO IN PILLOLE ISCRIZIONI IERATICHE NELLA TOMBA DI THUTMOSI IV Italiani in Egitto: Ernesto Schiaparelli | L’Arte di Shamira | I papiri di Carla BOLLETTINO INFORMATIVO DELL'ASSOCIAZIONE EGITTOLOGIA.NET NUMERO 3 e d i t o r i a l e La prolungata e precoce presenza di questo Confesso che questo numero di EM – Egitto- insolito e intenso caldo, dà l’impressione che logia.net Magazine è stato sul punto di non l’estate stia già volgendo al termine, anche se uscire! La prossimità con il ferragosto e il in realtà la legna accumulata per l’inverno caldo scoraggiante, soprattutto nelle due set- dovrà aspettare ancora molto tempo prima di timane centrali del mese di luglio – periodo in essere utile. cui il terzo numero del magazine ha comin- Curioso come hanno deciso di chiamare le tre ciato a prendere vita – ci avevano fatto propen- fasi più intense del caldo i meteorologici: Sci- dere per una sospensione, procrastinandone pione, Caronte e Minosse. Curioso perché mi l’uscita direttamente a ottobre. vien da pensare che l’epiteto “Africano” di Sci- Ma abbiamo resistito alla tentazione, sospen- pione e il collegamento con l’Ade che è possi- dendo solo una parte dei temi che abbiamo bile fare con Caronte e Minosse, abbia cominciato a trattare nei numeri precedenti, richiamato alla mente degli scienziati il con- come ci è stato richiesto dagli autori degli cetto di “caldo”. -

Minnowenvironmental Inc
--~------- minnowenvironmental inc. _____ 2 Lamb Street Georgetown, Ontario L7G 3M9 Memorandum To: Dan Cornett, Access Consulting Group From: Cynthia Russel, Minnow Environmental Inc. Date: February 13, 2008-02-13 Re: Update of Surface Water Quality Assessment for United Keno Hill Mine Complex. Minnow Environmental Inc. (Minnow) was retained by Access Consulting Group to undertake an assessment of the existing water quality data for the United Keno Hill Mine Complex (United Keno, Galena and Sourdough Hill). The objective of this assessment was to identify parameters and locations of concern within the downstream waters relative to established guidelines and background. This information, combined with toxicity data and watershed use objectives may then be combined to develop an approach for considering the development of Site Specific Water Quality Objectives (SSWQO) for various parameters and locations. In order to meet the study objectives, a progressive assessment of the available water quality data was undertaken which included the following steps; • Screen all data to identify outliers (i.e., those greater then 3 standard deviations from the mean) and remove these data. • Establish the background concentration for each parameter based the upper limit of background data distribution (mean + t S.D.) for the combined data from KV-1 and KV-37. UKH Surface Water Quality Assessment - Progress Report • Identify parameters with high method detection limits relative to guidelines which preclude determination of whether concentrations exceed the guideline. • Identify background concentrations which exceed the Canadian Water Quality Guidelines (CWQG). • Determine the median, mean, minimum and maximum concentration for each parameter at each location. • Determine which locations exceed background and/or CWQG at measurable (10%) and substantial (50%) frequencies. -
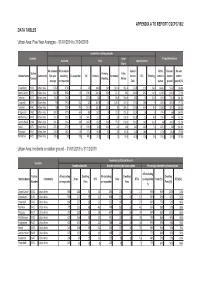
DSFRA IKEN Report Template
APPENDIX A TO REPORT CSCPC/19/2 DATA TABLES Urban Area: Five-Year Averages – 01/04/2014 to 31/04/2019 Incidents on station grounds Location False Pump Attendances Overview Fires Special Service Alarm All incidents All incidents Special All by On own On own Station Primary: False Station Name Community five-year excluding Co-responder All Primary Secondary Service RTC Flooding station's station station Number Dwelling Alarms average co-responder Calls pumps ground ground (%) Greenbank KV50 Urban Area 878.6 878.6 0 245 104.6 56.6 140.4 361.4 271.8 21.6 24.6 1424.8 974.2 68.4% Danes Castle KV32 Urban Area 832.6 830.8 1.8 198.8 126.4 56.6 72.4 385 248.4 29.2 14.8 1090.6 849.4 77.9% Torquay KV17 Urban Area 744.8 744.8 0 207.8 111 59 96.8 306.8 230 36 15.8 919.8 776.4 84.4% Crownhill KV49 Urban Area 742 741.8 0.2 227 100.6 43 126.4 337.4 177.4 28.6 9 878.4 680.6 77.5% Taunton KV61 Urban Area 734 733.4 0.6 227.8 132.8 56.6 95 284.6 221.6 65.4 8.4 1038.8 901.8 86.8% Bridgwater KV62 Urban Area 584.2 577.6 6.6 160 88.2 38 71.8 231.8 192.4 56 8 774.4 666 86.0% Middlemoor KV59 Urban Area 537.6 535.8 1.8 144.2 91.2 33 53 239.6 153.8 51 8.8 724.4 444 61.3% Camels Head KV48 Urban Area 491.6 491.2 0.4 162.8 85.2 50.4 77.6 178.6 150.2 16.6 11.8 638 390.2 61.2% Yeovil KV73 Urban Area 471.6 471.6 0 139.6 78.6 34.8 61 191 141 46.8 7.4 674.2 569 84.4% Plympton KV47 Urban Area 218.4 204.4 14 57.8 34.8 12 23 87.8 72.4 18.6 3 170.6 135.8 79.6% Plymstock KV51 Urban Area 185.8 185 0.8 48.4 27.4 12 21 76.8 60.6 12.6 2.6 165.4 123.8 74.8% Urban Area: Incidents on -

Expanding the Toolkit for Metabolic Engineering
Expanding the Toolkit for Metabolic Engineering Yao Zong (Andy) Ng Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2016 © 2016 Yao Zong (Andy) Ng All rights reserved ABSTRACT Expanding the Toolkit for Metabolic Engineering Yao Zong (Andy) Ng The essence of metabolic engineering is the modification of microbes for the overproduction of useful compounds. These cellular factories are increasingly recognized as an environmentally-friendly and cost-effective way to convert inexpensive and renewable feedstocks into products, compared to traditional chemical synthesis from petrochemicals. The products span the spectrum of specialty, fine or bulk chemicals, with uses such as pharmaceuticals, nutraceuticals, flavors and fragrances, agrochemicals, biofuels and building blocks for other compounds. However, the process of metabolic engineering can be long and expensive, primarily due to technological hurdles, our incomplete understanding of biology, as well as redundancies and limitations built into the natural program of living cells. Combinatorial or directed evolution approaches can enable us to make progress even without a full understanding of the cell, and can also lead to the discovery of new knowledge. This thesis is focused on addressing the technological bottlenecks in the directed evolution cycle, specifically de novo DNA assembly to generate strain libraries and small molecule product screens and selections. In Chapter 1, we begin by examining the origins of the field of metabolic engineering. We review the classic “design–build–test–analyze” (DBTA) metabolic engineering cycle and the different strategies that have been employed to engineer cell metabolism, namely constructive and inverse metabolic engineering. -

The West Valley May Harbor Two Impressive King Tombs, but It Is a Poor Cousin to the Valley of the Kings
Copyright © 2021 Michael J. Marfleet Published July 2nd, 2021 The West Valley may harbor two impressive king tombs, but it is a poor cousin to the Valley of the Kings. It will likely remain so. II. The West Valley 'Valley of the Aten' by MICHAEL J MARFLEET To date six 'tombs' have been discovered in the West Valley, (Figs. 1 & 2): KV22 - The extensive king tomb of Amenhotep III; KV23 - The king tomb of Ay, fore- shortened and perhaps half the size it was originally intended to be; KV24 - A pit/shaft 'tomb' located between the entrances to KV23 & KV25; KV25 - An un- finished king tomb never used for a king burial; KV65* and KVA, pit/shaft 'tombs' intended to house the sacred refuse from mummification and the funer- ary feast, (Technical Essay 1 appearing November 5th). The only two kings known to have been buried in the valley were both closely associated with the monotheistic Aten faith. Could the West Valley have been a separate necropolis set-aside exclus- ively for those of monotheistic belief; ie: the royals of the AMARNA Period? Amenhotep III and his father, Tuthmosis IV represent the earliest and Ay the latest of the pharaohs most directly associated with the Aten faith. The inter- vening kings, Amenhotep IV, Tutankhamun and Smenkhkare (Fig. 3), were buried elsewhere - Amenhotep IV in the Royal Wadi at Akhetaten (Fig. 4), and Tutankhamun in the Valley of the Kings (VoK). The whereabouts of the tomb of Smenkhkare, indeed the very existence of this king, remains a mystery, (Essay V, August 13th). -
![Mois Vie De Mozart Œuvres Novembre [18] Mozart Dirige La Cantate Maçonnique Laut Verkunde Unsre Freude (K.623) Pour L’Inaugura- Tion Du Nouveau Temple De Sa Loge](https://docslib.b-cdn.net/cover/7193/mois-vie-de-mozart-%C5%93uvres-novembre-18-mozart-dirige-la-cantate-ma%C3%A7onnique-laut-verkunde-unsre-freude-k-623-pour-l-inaugura-tion-du-nouveau-temple-de-sa-loge-2867193.webp)
Mois Vie De Mozart Œuvres Novembre [18] Mozart Dirige La Cantate Maçonnique Laut Verkunde Unsre Freude (K.623) Pour L’Inaugura- Tion Du Nouveau Temple De Sa Loge
Mois Vie de Mozart Œuvres Novembre [18] Mozart dirige la cantate maçonnique Laut verkunde unsre Freude (K.623) pour l’inaugura- tion du nouveau temple de sa loge. [20] Mozart doit s’aliter. [28] Ses médecins, Thomas Franz Closset et Matthia von Sallaba, s’interrogent sur l’état réel de leur pa- tient – l’avenir le leur dira assez clairement –. Décembre [4] Lecture des parties Requiem en ré mineur, déjà achevées du Requiem, K.626 (inachevé) alors qu’il ne lui reste qu’une quinzaine d’heures à vivre. C’est lui-même qui chante la partie d’alto, puis il donne à Süssmayr des indications pour termi- ner l’ouvrage. Se savait-il mourant ou voulait-il, plus simplement, que la commande soit honorée dans les temps, le lecteur choisira. Il reste lucide toute la soirée. [5] Mozart s’éteint à 0h55. [6] Après une bénédiction devant la Chapelle du Crucifix de Saint-Etienne, Mozart est enterré dans la fosse commune du cime- tière St. Marx. 1792 Mois Vie de Mozart Œuvres Janvier [27] Mozart aurait eu trente-six ans… 240 1 Mois Vie de Mozart Œuvres Mai [9] …décrète géné- [4] Andante en fa majeur reusement que Mozart pour un petit orgue mécani- sera adjoint (bénévole que, K.616 non-rémunéré) du célèbre Kapellmeister Hofmann [23] Adagio et Rondo pour de la cathédrale et qu’il Glassharmonika, flûte, pourra occuper le poste de hautbois, alto et violoncelle, titulaire dès que celui-ci « K.617 sera libéré » à la mort ou la retraite de Hofmann. Juin [4] Constance, accompa- [17] Motet en ré majeur gnée de son fils Karl, se «Ave verum Corpus», K.618 rend à nouveau en cure à Baden.