A Model Scenario for Kieserite-Dominated Evaporites in Juventae Chasma, Mars
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evidence for Volcanism in and Near the Chaotic Terrains East of Valles Marineris, Mars
43rd Lunar and Planetary Science Conference (2012) 1057.pdf EVIDENCE FOR VOLCANISM IN AND NEAR THE CHAOTIC TERRAINS EAST OF VALLES MARINERIS, MARS. Tanya N. Harrison, Malin Space Science Systems ([email protected]; P.O. Box 910148, San Diego, CA 92191). Introduction: Martian chaotic terrain was first de- ple chaotic regions are visible in CTX images (Figs. scribed by [1] from Mariner 6 and 7 data as a “rough, 1,2). These fractures have widened since the formation irregular complex of short ridges, knobs, and irregular- of the flows. The flows overtop and/or bank up upon ly shaped troughs and depressions,” attributing this pre-existing topography such as crater ejecta blankets morphology to subsidence and suggesting volcanism (Fig. 2c). Flows are also observed originating from as a possible cause. McCauley et al. [2], who were the fractures within some craters in the vicinity of the cha- first to note the presence of large outflow channels that os regions. Potential lava flows are observed on a por- appeared to originate from the chaotic terrains in Mar- tion of the floor as Hydaspis Chaos, possibly associat- iner 9 data, proposed localized geothermal melting ed with fissures on the chaos floor. As in Hydraotes, followed by catastrophic release as the formation these flows bank up against blocks on the chaos floor, mechanism of chaotic terrain. Variants of this model implying that if the flows are volcanic in origin, the have subsequently been detailed by a number of au- volcanism occurred after the formation of Hydaspis thors [e.g. 3,4,5]. Meresse et al. -

Aqueous Minerals from Arsia Chasmata of Arsia Mons, Tharsis Region: Implications for Aqueous Alteration Processes on Mars
45th Lunar and Planetary Science Conference (2014) 1826.pdf AQUEOUS MINERALS FROM ARSIA CHASMATA OF ARSIA MONS, THARSIS REGION: IMPLICATIONS FOR AQUEOUS ALTERATION PROCESSES ON MARS. N. Jain*, S. Bhattacharya, P. Chauhan, Space Applications Centre (ISRO), Ahmedabad, Gujarat, India ([email protected]/ Fax: +91-079- 26915825). Introduction: The Arsia Chasmata is a complex help of high resolution data such as MGS-MOC (Mars collapsed region located at the northeastern flank of Global Surveyor-Mars Orbiter Camera), Viking orbiter Arsia Mons (figure 1 A and B) within Tharsis region [3], fresh appearing lava flows [4], graben and glaciers of planet Mars and is the most important region for the on flanks of Arsia Mons [5], young lava flows [6] study of minerals like phyllosilicate and pyroxene. The small shields at floor of caldera [7]. reflectance data of MRO-CRISM (figure 1 C D) has Present study mainly focuses on the mineralogy of confirmed above mentioned minerals in the study area. Arsia Chasmata which interestingly contains The presence of these minerals at the Arsia Chasmata absorption features of aqueous altered minerals such as on Mars provides the evidence of its past watery serpentine (phyllosilicate). This mineral is also located environment and their processes of formation. In the in Nili Fossae region which is long, narrow depression present study the absorption features of serpentine present on Mars [8]. But in the present study (phyllosilicate) are obtained at 2.32 µm, 1.94 µm and occurrence of this mineral at high altitude region raise 2.51 µm. Previous studies on Mars show that the curiosity to know about their formation processes. -

Mineralogy of the Martian Surface
EA42CH14-Ehlmann ARI 30 April 2014 7:21 Mineralogy of the Martian Surface Bethany L. Ehlmann1,2 and Christopher S. Edwards1 1Division of Geological & Planetary Sciences, California Institute of Technology, Pasadena, California 91125; email: [email protected], [email protected] 2Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California 91109 Annu. Rev. Earth Planet. Sci. 2014. 42:291–315 Keywords First published online as a Review in Advance on Mars, composition, mineralogy, infrared spectroscopy, igneous processes, February 21, 2014 aqueous alteration The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: The past fifteen years of orbital infrared spectroscopy and in situ exploration 10.1146/annurev-earth-060313-055024 have led to a new understanding of the composition and history of Mars. Copyright c 2014 by Annual Reviews. Globally, Mars has a basaltic upper crust with regionally variable quanti- by California Institute of Technology on 06/09/14. For personal use only. All rights reserved ties of plagioclase, pyroxene, and olivine associated with distinctive terrains. Enrichments in olivine (>20%) are found around the largest basins and Annu. Rev. Earth Planet. Sci. 2014.42:291-315. Downloaded from www.annualreviews.org within late Noachian–early Hesperian lavas. Alkali volcanics are also locally present, pointing to regional differences in igneous processes. Many ma- terials from ancient Mars bear the mineralogic fingerprints of interaction with water. Clay minerals, found in exposures of Noachian crust across the globe, preserve widespread evidence for early weathering, hydrothermal, and diagenetic aqueous environments. Noachian and Hesperian sediments include paleolake deposits with clays, carbonates, sulfates, and chlorides that are more localized in extent. -

1469 Vol 43#5 Art 03.Indd
1469 The Canadian Mineralogist Vol. 43, pp. 1469-1487 (2005) BORATE MINERALS OF THE PENOBSQUIS AND MILLSTREAM DEPOSITS, SOUTHERN NEW BRUNSWICK, CANADA JOEL D. GRICE§, ROBERT A. GAULT AND JERRY VAN VELTHUIZEN† Research Division, Canadian Museum of Nature, P.O. Box 3443, Station D, Ottawa, Ontario K1P 6P4, Canada ABSTRACT The borate minerals found in two potash deposits, at Penobsquis and Millstream, Kings County, New Brunswick, are described in detail. These deposits are located in the Moncton Subbasin, which forms the eastern portion of the extensive Maritimes Basin. These marine evaporites consist of an early carbonate unit, followed by a sulfate, and fi nally, a salt unit. The borate assemblages occur in specifi c beds of halite and sylvite that were the last units to form in the evaporite sequence. Species identifi ed from drill-core sections include: boracite, brianroulstonite, chambersite, colemanite, congolite, danburite, hilgardite, howlite, hydroboracite, kurgantaite, penobsquisite, pringleite, ruitenbergite, strontioginorite, szaibélyite, trembathite, veatchite, volkovskite and walkerite. In addition, 41 non-borate species have been identifi ed, including magnesite, monohydrocalcite, sellaite, kieserite and fl uorite. The borate assemblages in the two deposits differ, and in each deposit, they vary stratigraphically. At Millstream, boracite is the most common borate in the sylvite + carnallite beds, with hilgardite in the lower halite strata. At Penobsquis, there is an upper unit of hilgardite + volkovskite + trembathite in halite and a lower unit of hydroboracite + volkov- skite + trembathite–congolite in halite–sylvite. At both deposits, values of the ratio of B isotopes [␦11B] range from 21.5 to 37.8‰ [21 analyses] and are consistent with a seawater source, without any need for a more exotic interpretation. -

Evolution of the Astonishing Naica Giant Crystals in Chihuahua, Mexico
minerals Review Evolution of the Astonishing Naica Giant Crystals in Chihuahua, Mexico Iván Jalil Antón Carreño-Márquez 1 , Isaí Castillo-Sandoval 2, Bernardo Enrique Pérez-Cázares 3, Luis Edmundo Fuentes-Cobas 2 , Hilda Esperanza Esparza-Ponce 2 , Esperanza Menéndez-Méndez 4, María Elena Fuentes-Montero 3 and María Elena Montero-Cabrera 2,* 1 Department of Engineering, Universidad La Salle Chihuahua, Chihuahua 31625, Mexico; [email protected] 2 Department of Environment and Energy, Centro de Investigación en Materiales Avanzados, Chihuahua 31136, Mexico; [email protected] (I.C.-S.); [email protected] (L.E.F.-C.); [email protected] (H.E.E.-P.) 3 Department of Computational Chemistry, Universidad Autónoma de Chihuahua, Chihuahua 31125, Mexico; [email protected] (B.E.P.-C.); [email protected] (M.E.F.-M.) 4 Department Physicochemical Assays, Instituto Eduardo Torroja de Ciencias de la Construcción, 28033 Madrid, Spain; [email protected] * Correspondence: [email protected] Abstract: Calcium sulfate (CaSO4) is one of the most common evaporites found in the earth’s crust. It can be found as four main variations: gypsum (CaSO4·2H2O), bassanite (CaSO4·0.5H2O), soluble Citation: Carreño-Márquez, I.J.A.; anhydrite, and insoluble anhydrite (CaSO4), being the key difference the hydration state of the Castillo-Sandoval, I.; Pérez-Cázares, sulfate mineral. Naica giant crystals’ growth starts from a supersaturated solution in a delicate B.E.; Fuentes-Cobas, L.E.; Esparza- thermodynamic balance close to equilibrium, where gypsum can form nanocrystals able to grow Ponce, H.E.; Menéndez-Méndez, E.; up to 11–12 m long. -

An Overview of Morphological and Compositional Investigation of Melas Chasma of Valles Marineris
Ninth International Conference on Mars 2019 (LPI Contrib. No. 2089) 6145.pdf An Overview of Morphological and Compositional investigation of Melas Chasma of Valles Marineris. T. Sivasankari1 and S. Arivazhagan2, 1 2 Centre For Applied Geology, Gandhigram Rural Institute - Deemed to be University, Gandhigram, Dindigul-624302, India ([email protected] and [email protected]). Introduction Many studies had been carried out by the primarily by aeolian processes in the recent period of time. researchers for various region of Valles Marineris and has Fig. 2. Shows the geology map of the study area. The geolo- revealed evidence for the presence of sulphate deposits gy map of Melas chasma was derived based on the map giv- namely the Interior Layered Deposits (ILD). The present en by Witbeck et al, 1991. The geological features includes study aims at the identification of mafic minerals High Cal- slope materials on the valley walls, dunes, layered materials, cium Pyroxene [HCP] and Phyllosilicate minerals of Melas fractured sediments, ridged plains, floor materials and crater chasma region of Valles Marineris by using CRISM datasets. fill materials. The topography of Chasma was interpreted by The spectra and the mineral distribution maps are derived the contuor map and DEM derived using the MOLA data. using the spectral summary parameters which helps to under- The contour values shows the highest relief of the study area stand the past geologic events ultimately bringing out the is 4000m and the lowest relief is -3800m. From the DEM origin and evolution of Valles Marineris. The Morphological data it is evident that the chasma has almost smooth valley features of Valles Marineris are compared with the CRISM floor with small isolated patches and a rugged topography as well as the HiRiSE Data. -

The Mineralogy of the British Permian Evaporites
460 The mineralogy of the British Permian evaporites By F. H. STEWART Department of Geology, University of Edinburgh Summary. The occurrence of the minerals is reviewed. Consideration of their genesis leads to a tentative list of primary minerals, and of early diagenetie, later diagenetic and geothermal metamorphic, and late near-surface changes. N 1943 Professor Tilley recorded the occurrence of po]yhalite in the I rocks of the D'Arcy Exploration Company's E.2 borehole at Aislaby, near Whitby; this was the first published record of a potassium-bearing salt in British evaporites. The occurrence of rocks containing sylvine (Lees and Taitt, 1946) prompted further exploration by Imperial Chemical Industries and Fisons Ltd. after the war, and this showed the presence of large deposits of potassium ores in the Whitby region. There is now a considerable literature related to these, and it seems timely to give a short review of the mineralogy of the British Permian evaporites. The writer is most grateful to Fisons Ltd. who have kindly allowed him to use unpublished information from his study of their boreholes F.1 at Robin Hood's Bay, F.2 at Staintondale, F.3 at Little Beck, and F.4 at Hawsker, in the Whitby Scarborough district. The position of these and the other boreholes mentioned in this paper can be found in the map of Dunham (1960). Dist~'ibution of the minerals The evaporites occur in two principal areas east and west of the Pennine Hills. In the western area (the Vale of Eden, west Cumberland, and the Furness district of Lancashire), where four evaporite beds have been recognized (Hollingworth, 1942), the mineralogy is relatively simple; carbonates, anhydrite, and gypsum are the main constituents. -
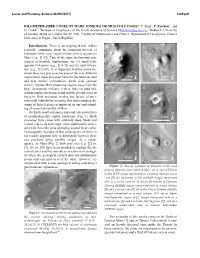
KILOMETER-SIZED CONES on MARS: IGNEOUS OR MUD VOLCANOES?. P. Brož1, P. Fawdon2, and O. Čadek3, 1Institute of Geophysics Of
Lunar and Planetary Science XLVIII (2017) 1425.pdf KILOMETER-SIZED CONES ON MARS: IGNEOUS OR MUD VOLCANOES?. P. Brož1, P. Fawdon2, and O. Čadek3, 1Institute of Geophysics of the Czech Academy of Science ([email protected]), 2Birkbeck, University of London, Malet St, London WC1E 7HX, 3Faculty of Mathematics and Physics, Department of Geophysics, Charles University in Prague, Czech Republic. Introduction: There is an ongoing debate within scientific community about the formation process of kilometer-sized cones reported from several regions on Mars (e.g., [1-8]). Two of the main mechanisms con- sidered as possible explanations, are (1) small scale igneous volcanism (e.g., [1,4-7]) and (2) mud volcan- ism (e.g., [2,8,10]). It is important to differentiate be- tween these two processes because of the very different implications these processes have for the Martian crust and near surface environment. Small scale igneous activity implies Mars producing magma away from the large Amazonian volcanic centers, whereas mud vol- canism implies pressurized and mobile ground water in the past. Both processes involve key factors of envi- ronmental habitability meaning that understanding the origin of these features is important in our understand- ing of recent habitability of Mars. On Earth small volcanoes and mud volcanism form of morphologically similar landforms (Fig. 1). Both processes form cones with relatively steep flanks and central craters on their tops; often additionally associ- ated with flow-like units spreading around those cones. Consequently, because of this convergence of form, is not readily apparent how to distinguish between these two processes using satellite images. -

Crystallization of Kainite from Solutions in Syste
ineering ng & E P l r a o c i c e m s e s Journal of h T C e f c h o Kostiv and Basystiuk, J Chem Eng Process Technol 2016, 7:3 l ISSN: 2157-7048 n a o n l o r g u y o J Chemical Engineering & Process Technology DOI: 10.4172/2157-7048.1000298 Research Article Article OpenOpen Access Access + 2+ + - 2- Crystallization of Kainite from Solutions in System K , Mg , Na // Cl , SO4 -Н2О Kostiv IY1 and Yа І Basystiuk2* 1State Enterprise Scientific - Research Gallurgi Institute 5a, Fabrichna Str., 76000 Kalush, Ukraine 2Precarpathian National University named after V. Stephanyk 57, Shevchenko Str., 76025 Ivano-Frankivsk, Ukraine Abstract In isothermal conditions have been studied the influence of system solution sea salts during their evaporation and crystallization to composition of received liquid and solid phases. It is shown that evaporation of the solution leading 2- to its supersaturation by sulfate salts. In the liquid phase before crystallization of kainite concentration of SO4 ions increases to 10% and above. Through this probability of formation of crystals increases and the result of evaporation 2- is the formation of finely dispersed kainite. During crystallization of kainite concentration of SO4 in the liquid phase 2- 2+ decreases rapidly. Decreasing its intensity increases with increasing value k=E SO4 : E Mg of initial solution. For 2- the degree of evaporation of 20.0% and 31.0% concentration of SO4 in evaporated solution on the value of k does not change. Most forms of potassium salts in the solid phase for the value k=0.7573 and reaches a maximum value at 82% evaporation rate of 31%. -

Mechanism and Kinetics of Dehydration of Epsomite Crystals Formed in the Presence of Organic Additives
J. Phys. Chem. B 2007, 111, 41-52 41 Mechanism and Kinetics of Dehydration of Epsomite Crystals Formed in the Presence of Organic Additives Encarnacio´n Ruiz-Agudo,† J. Daniel Martı´n-Ramos,‡ and Carlos Rodriguez-Navarro* Departamento de Mineralogı´a y Petrologı´a, UniVersidad de Granada, FuentenueVa s/n, 18002 Granada, Spain ReceiVed: July 14, 2006; In Final Form: October 10, 2006 The thermal dehydration of epsomite (MgSO4‚7H2O) crystals grown in the presence and absence of organic additives (phosphonates, carboxylic acids, and polyacrylic acid derivatives) was studied by means of thermogravimetry (TG), differential scanning calorimetry (DSC), X-ray thermodiffraction (XRTD), and environmental scanning electron microscopy (ESEM). In situ XRTD analyses (in air, 30% relative humidity) show an epsomite f hexahydrite (MgSO4‚6H2O) transition at 25-38 °C, followed by formation of amorphous phase(s) at T > 43-48 °C, and MgSO4 crystallization at ∼300 °C. Kinetic parameters (ER and A) were determined for the main dehydration step (25-160 °C), which corresponds to a MgSO4‚7H2O f MgSO4‚ H2O transition, by applying two isoconversional methods to nonisothermal TG data obtained at different heating rates (â ) 1, 3, and 5 K‚min-1). In situ, hot-stage ESEM observations of the thermal dehydration of epsomite crystals are consistent with the nonisothermal kinetic study and, along with XRTD results, allow us to propose a dehydration mechanism which includes an early nucleation and growth event, followed by the advancement of the reaction interface (3D phase boundary reaction). Both ER and A values increase in the presence of the most effective crystallization inhibitors tested. -

Possible Evaporite Karst in an Interior Layered Deposit in Juventae
International Journal of Speleology 46 (2) 181-189 Tampa, FL (USA) May 2017 Available online at scholarcommons.usf.edu/ijs International Journal of Speleology Off icial Journal of Union Internationale de Spéléologie Possible evaporite karst in an interior layered deposit in Juventae Chasma, Mars Davide Baioni* and Mario Tramontana Planetary Geology Research Group, Dipartimento di Scienze Pure e Applicate (DiSPeA), Università degli Studi di Urbino "Carlo Bo", Campus Scientifico Enrico Mattei, 61029 Urbino (PU), Italy Abstract: This paper describes karst landforms observed in an interior layered deposit (ILD) located within Juventae Chasma a trough of the Valles Marineris, a rift system that belongs to the Tharsis region of Mars. The ILD investigated is characterized by spectral signatures of kieserite, an evaporitic mineral present on Earth. A morphologic and morphometric survey of the ILD surface performed on data of the Orbiter High Resolution Imaging Science Experiment (HiRISE) highlighted the presence of depressions of various shapes and sizes. These landforms interpreted as dolines resemble similar karst landforms on Earth and in other regions of Mars. The observed karst landforms suggest the presence of liquid water, probably due to ice melting, in the Amazonian age. Keywords: Mars, interior layered deposits, karst, climate change Received 21 Octomber 2016; Revised 18 April 2017; Accepted 19 April 2017 Citation: Baioni D. and Tramontana M., 2017. Possible evaporite karst in an interior layered deposit in Juventae Chasma, Mars. International Journal of Speleology, 46 (2), 181-189. Tampa, FL (USA) ISSN 0392-6672 https://doi.org/10.5038/1827-806X.46.2.2085 INTRODUCTION north of Valles Marineris (Catling et al., 2006). -

Stability of Magnesium Sulfate Minerals in Martian Environments
Lunar and Planetary Science XXXVI (2005) 2290.pdf STABILITY OF MAGNESIUM SULFATE MINERALS IN MARTIAN ENVIRONMENTS. G.M. Marion1 and J.S. Kargel2, 1Desert Research Institute, 2215 Raggio Parkway, Reno, NV 89512, [email protected], 2U.S. Geological Survey, 2255 N. Gemini Dr., Flagstaff, AZ 86001, [email protected]. Introduction: Viking Lander, Pathfinder, and –3.6°C for a pure MgSO4-H2O system. In this system, Mars Exploration Rover missions to Mars have found the lower hydrates, hexahydrite and kieserite, only abundant sulfur in surface soils and rocks, and the best form at higher temperatures (48°C and 69°C, respec- indications are that magnesium sulfates are among the tively). key hosts [1-6]. At Meridiani Planum, MgSO4 salts The presence of other constituents in ternary (or constitute 15 to 40 wt.% of sedimentary rocks [3-5,7]. higher) systems can lower the temperatures at which Additional S is hosted by gypsum and jarosite. Reflec- lower hydrates are stable. For example, hexahydrite is tance and thermal emission spectroscopy is consistent stable at 25°C in a MgSO4-MgCl2-H2O system at 3.8 with the presence of kieserite (MgSO4•H2O) and ep- m MgCl2 [12]. This requires high chloride concentra- somite (MgSO4•7H2O) [3]. Theoretically, the tions that may occur in Martian environments that dodecahydrate (MgSO4•12H2O) should also have have previously undergone extensive sulfate precipita- precipitated [8]. tion and chloride concentration due to extreme freez- We first examine theoretically which MgSO4 min- ing or desiccating conditions [3-5]. erals should have precipitated on Mars, and then how Based on the Rover mission to Meridiani Planum, dehydration might have altered these minerals.