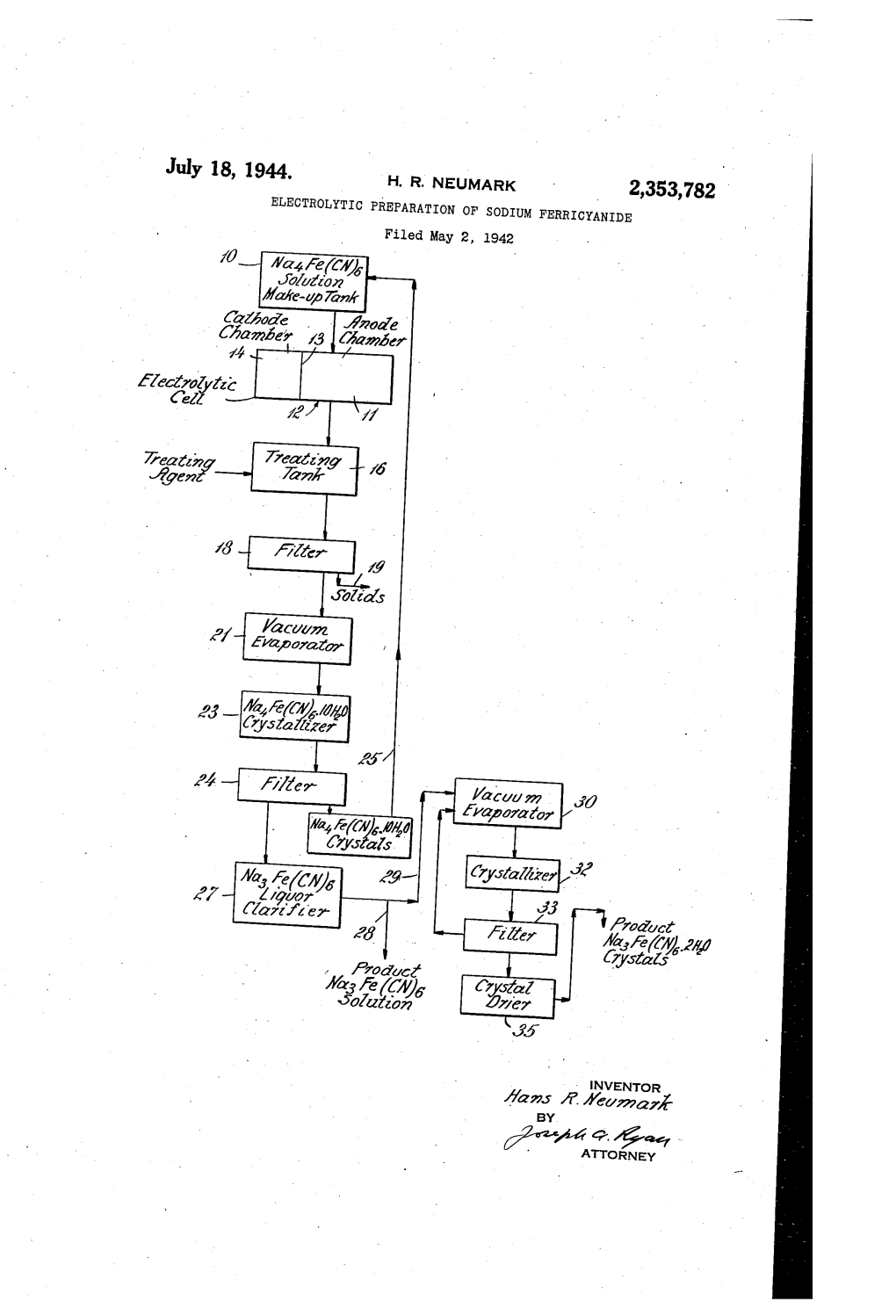
ELECTROLYTIC PREPARATION of SODIUM FERRICYANIDE Filed May 2, 1942
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Piccolo® General Chemistry 13
Piccolo® General Chemistry 13 For In Vitro Diagnostic Use and For Professional Use Only Customer and Technical Service: 1-800-822-2947 Applicable to US customers only Customers outside the US: +49 6155 780 210 CLIA Waived: Use lithium heparin whole blood, only Moderate Complexity: Use lithium heparin whole blood, lithium heparin plasma, or serum Abaxis, Inc. ABAXIS Europe GmbH 3240 Whipple Rd. Bunsenstr. 9-11 Union City, CA 94587 64347 Griesheim USA Germany 1. Intended Use The Piccolo® General Chemistry 13 used with the Piccolo blood chemistry analyzer or the Piccolo Xpress® chemistry analyzer, is intended to be used for the in vitro quantitative determination of alanine aminotransferase (ALT), albumin, alkaline phosphatase (ALP), amylase, aspartate aminotransferase (AST), calcium, creatinine, gamma glutamyltransferase (GGT), glucose, total bilirubin, total protein, blood urea nitrogen (BUN), and uric acid in heparinized whole blood, heparinized plasma, or serum in a clinical laboratory setting or point-of-care location. For US Customers Only The tests on this panel are waived under CLIA ’88 regulations. If a laboratory modifies the test system instructions, then the tests are considered high complexity and subject to all CLIA requirements. For CLIA waived labs, only lithium heparin whole blood may be tested. For use in moderate complexity labs, lithium heparinized whole blood, lithium heparinized plasma, or serum may be used. A CLIA Certificate of Waiver is needed to perform CLIA waived testing. A Certificate of Waiver can be obtained from the Centers for Medicare & Medicaid Services (CMS). Please contact the Commission on Laboratory Accreditation (COLA) at 1- 800-981-9883 for assistance in obtaining one. -

Excess Heat Production in the Redox Couple Reaction of Ferricyanide and Ferrocyanide
www.nature.com/scientificreports OPEN Excess heat production in the redox couple reaction of ferricyanide and ferrocyanide Atsushi Sugiyama1,2,3*, Makoto Miura4, Yoshinobu Oshikiri5, Yena Kim3, Ryoichi Morimoto6, Miki Miura7, Tetsuya Osaka2, Iwao Mogi8, Yusuke Yamauchi3,9* & Ryoichi Aogaki3,10* In order to establish the universality of the excess heat production in electrochemical reaction, under a high magnetic feld, as one of the most fundamental electrochemical reactions, the case of ferricyanide-ferrocyanide redox reaction was examined, where ionic vacancies with ± 1 unit charge were collided by means of magnetohydrodynamic (MHD) fow. As a result, from the pair annihilation of the vacancies with opposite signs, beyond 7 T, excess heat production up to 25 kJ·mol−1 in average at 15 T was observed, which was attributed to the liberation of the solvation energy stored in a pair of the vacancy cores with a 0.32 nm radius, i.e., 112 kJ·mol−1. Diference between the observed and expected energies comes from the small collision efciency of 0.22 due to small radius of the vacancy core. Ionic vacancy initially created as a by-product of electrode reaction is unstable in solution phase, stabilized by releasing solvation energy. Ionic vacancy utilizes the energy to enlarge the core and stores the energy in it. As a result, solvated ionic vacancy consists of a polarized free space of the enlarged core surrounded by oppositely charged ionic cloud. The accuracy and precision of the measured values were ascertained by in situ standard additive method. Recently, in copper redox reaction under a high magnetic feld, great excess heat production up to 410 kJ·mol−1 in average has been observed, which is 1.5 times larger than the molar combustion heat of hydrogen (285.8 kJ·mol−1)1. -

Re‐Evaluation of Sodium Ferrocyanide (E
SCIENTIFIC OPINION ADOPTED: 29 June 2018 doi: 10.2903/j.efsa.2018.5374 Re-evaluation of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536) and calcium ferrocyanide (E 538) as food additives EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Maged Younes, Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipic, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert-Remy, Gunter Georg Kuhnle, Claude Lambre, Jean-Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens-Berendsen, Matthew Wright, Alessandro Di Domenico, Henk Van Loveren, Alessandra Giarola, Zsuzsanna Horvath, Federica Lodi and Rudolf Antonius Woutersen Abstract The Panel on Food Additives and Nutrient Sources added to Food (ANS) provided a scientific opinion re-evaluating the safety of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536), and evaluating the safety of calcium ferrocyanide (E 538) as food additives. The Panel considered that adequate exposure and toxicity data were available. Ferrocyanides (E 535–538) are solely authorised in two food categories as salt substitutes. To assess the dietary exposure to ferrocyanides (E 535–538) from their use as food additives, the exposure was calculated based on regulatory maximum level exposure assessment scenario (maximum permitted level (MPL)) and the refined exposure assessment scenario. Dietary exposure to ferrocyanides was calculated based on mean and high levels consumption of salts in both the regulatory maximum level and the refined scenario. In the MPL scenario, the exposure to ferrocyanides (E 535–538) from their use as a food additive was up to 0.009 mg/kg body weight (bw) per day in children and adolescents. -

Reversibility of Ferri-/Ferrocyanide Redox During Operando Soft X-Ray Spectroscopy
Reversibility of Ferri-/Ferrocyanide Redox during Operando Soft X-ray Spectroscopy The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Risch, Marcel, Kelsey A. Stoerzinger, Tom Z. Regier, Derek Peak, Sayed Youssef Sayed, and Yang Shao-Horn. “Reversibility of Ferri-/ Ferrocyanide Redox During Operando Soft X-Ray Spectroscopy.” The Journal of Physical Chemistry C 119, no. 33 (August 20, 2015): 18903–18910. As Published http://dx.doi.org/10.1021/acs.jpcc.5b04609 Publisher American Chemical Society (ACS) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/109590 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Reversibility of Ferri-Ferrocyanide Redox During Operando Soft X-Ray Spectroscopy ┴ Marcel Risch,†* Kelsey A. Stoerzinger,§ Tom Z. Regier,‡ Derek Peak,º Sayed Youssef Sayed,† Yang Shao-Horn†§||* †Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, MA, USA 02139 §Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA 02139 ||Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA 02139 ‡Canadian Light Source, Inc., Saskatoon, SK, Canada S7N 2V3 ºDepartment of Soil Sciences, University of Saskatchewan, Saskatoon, SK, Canada S7N 5A8 KEYWORDS. Electrochemistry; In-situ; redox shuttle; iron cyanide; X-ray absorption; radiolysis; radiation damage. ABSTRACT The ferri-ferrocyanide redox couple is ubiquitous in many fields of physical chemistry. We studied its photochemical response to intense synchrotron radiation by in-situ X-Ray absorption spectroscopy. -

National Food Safety Standard on Sodium Ferrocyanide China
THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY Voluntary - Public Date: 2/2/2012 GAIN Report Number: China - Peoples Republic of Post: Beijing National Food Safety Standard on Sodium Ferrocyanide Report Categories: FAIRS Subject Report Approved By: Scott Sindelar Prepared By: M. Melinda Meador and Ma Jie Report Highlights: On November 17, 2011, China notified the WTO of National Food Safety Standard: Food Additive Sodium Ferrocyanide as SPS/N/CHN/485. This standard applies to food additive sodium ferrocyanide made from sodium cyanide and ferrous sulfate or ferrum reductum, sodium hydroxide and hydrogen cyanide. It specifies the scope, technical requirements and testing methods for food additive sodium ferrocyanide. The date for submission of final comments to China is January 16, 2012. The proposed date of entry is to be determined. Comments can be sent to China’s SPS Enquiry Point at [email protected]. This report is an INFORMAL translation of this document. General Information: BEGIN TRANSLATION GB National Food Safety Standard GB XXXX XXXX National Food Safety Standard Food Additive Sodium Ferrocyanide (Draft for Soliciting Opinions) Date of Issue: XXXX-XX-XX Date of Effectiveness: XXXX-XX-XX Issued by the Ministry of Health of the People’s Republic of China National Food Safety Standard Food Additive Sodium Ferrocyanide 1. Scope This standard applies to food additive sodium ferrocyanide made from sodium cyanide and ferrous sulfate or ferrum reductum, sodium hydroxide and hydrogen cyanide. 2. Molecular formula, constitutional formula and relative molecular mass 2.1 Molecular formula Na4Fe CN 6·10H2O 2.2 Relative molecular mass 484. -

No, Potassium Ferrocyanide in Some Salt Brands Is Not at Toxic Levels
Fact check: No, potassium ferrocyanide in some salt brands is not at toxic levels Shubashree Desikan JULY 02, 2019 Citing a report from U.S.-based West American Analytical Laboratories, Shiv Shankar Gupta, chairman of Godhum Grains and Food Products, accused some salt manufacturers in India of selling food-grade salt that contained high levels of potassium ferrocyanide. This caused a panic, which was aggravated by widespread sharing of the news item on social media. Indeed, potassium ferrocyanide is being used in salt to give it anti-caking properties. But the answer to whether it is toxic is no! Firstly, while potassium cyanide is a toxic substance and releases the cyanide anion when consumed by a person, potassium ferrocyanide is not. In potassium ferrocyanide, the cyanide anion is strongly bonded to ferrous ion and hence does not get hydrolysed. An image widely shared in social media. Secondly, there is the factor known as the LD50 value. This is expanded as “Lethal Dose 50%”. LD50 is the amount of any chemical that can cause death in 50% of the group of animals that it enters by consumption or absorption through the skin. According to inorganic chemist Dr. Sayam Sengupta of IISER Kolkata, the LD50 value for common salt — sodium chloride — for rats is greater than 3 gram per kilogram body weight of the animal. He points out that for potassium Ferrocyanide, LD 50 value is 3.6 gram per kilogram weight of the animal, when taken orally. As this indicates, the LD50 value of potassium ferrocyanide is almost the same as common salt. -

On the Cyanogen Halides by P
ON THE CYANOGEN HALIDES BY P. KAILASAM (From the Chemistry Department, Madras Christian College) Received July 31, 1941 (Communicated by Sir C. V. Raman, xt., F.R.S., NN.L.) I. Action of Halogens on Cyanogen Halides No observations appear to have been recorded on the action of halogens on cyanogen halides except that of chlorine on cyanogen chloride (Wurtz, 1851; Naumann and Vogt, 1870) and bromine on cyanogen bromide in ether (Poonamarew, 1885) which polymerise them to cyanuric chloride and cyanuric bromide respectively. The object of the present investigation is to study the possible displacement reactions with the cyanogen halides. The action of chlorine gas on cyanogen iodide and the action of bromine on cyanogen chloride were first studied. It was expected that chlorine would displace iodine from cyanogen iodide forming cyanogen chloride, but it was found that cyanogen iodide was not affected by dry chlorine. Dry cyanogen iodide, prepared by warming an intimate mixture of one part of iodine and two parts of AgCN, at about 40°, was sublimed into a narrow tube which could be heated in a water-bath. Dry chlorine was passed through the tube for nearly two hours. No immediate reaction occurred, and even after leaving for a week at room temperature (30°) the white needles of cyanogen iodide remained unchanged. The tube was then slowly heated, but no reaction occurred up to the sublimation temperature of cyanogen iodide (about 40°). It was found that cyanogen iodide could be sublimed unchanged in an atmosphere of dry chlorine. Since chlorine does not displace iodine from cyanogen iodide, it was thought that bromine might displace chlorine from cyanogen chloride form- ing cyanogen bromide. -

Research on Best Practices for Winter Weather Operations
Research on Best Practices for Winter Weather Operations Technical Report 0-6669-1 Cooperative Research Program PRAIRIE VIEW A&M UNIVERSITY PRAIRIE VIEW, TEXAS TEXAS A&M TRANSPORTATION INSTITUTE COLLEGE STATION, TEXAS in cooperation with the Federal Highway Administration and the Texas Department of Transportation http://tti.tamu.edu/documents/0-6669-1.pdf Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. FHWA/TX-12/0-6669-1 4. Title and Subtitle 5. Report Date RESEARCH ON BEST PRACTICES FOR WINTER WEATHER December 2011 OPERATIONS Published: October 2012 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Judy A. Perkins, Judith Mwakalonge, Debbie Jasek, Jodi Carson, Report 0-6669-1 Kwaku Obeng-Boampong, and Geza Pesti 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Prairie View A&M University & Texas A&M Transportation Institute 11. Contract or Grant No. Prairie View, Texas 77446 Project 0-6669 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered Texas Department of Transportation Technical Report: Research and Technology Implementation Office November 2010–October 2011 P.O. Box 5080 14. Sponsoring Agency Code Austin, Texas 78763-5080 15. Supplementary Notes Project performed in cooperation with the Texas Department of Transportation Project Title: Best Practices for Emergency Operations URL: http://tti.tamu.edu/documents/0-6669-1.pdf 16. Abstract There is a growing need to identify actionable practices relative to winter weather operations. Because of the potential and inherent hazards during cold weather, it has become increasingly important to ensure that these practices can be effectively employed as well as protect the health and safety of employees working in extreme conditions. -

HOW TO: Salt and Preservatives in Organic Food
What should I know about using salt and preservatives in organic food? Salt plays an important role in processed food products both for its flavoring properties and as a preservative. For those reasons both salt and sea salt are allowed in organic products as long as the salt or sea salt does not have any prohibited additives. Here we discuss the use of salt, prohibited additives, and answer common questions. Are additives allowed in the salt I use in my organic products? Though salt and sea salt are allowed in all categories of organic products, anti-caking/free-flow agents and whiteners are generally not allowed in these products. Common anti-caking/free- flow agents that are not allowed include: • Calcium silicate • Ferric ammonium citrate • Sodium ferrocyanide • Magnesium silicate • Magnesium carbonate • Propylene glycol • Aluminum calcium silicate • Sodium aluminosilicate Makers of organic products must be able to show that the salt does not contain any of the above flow agents, anti-caking agents, or any whiteners. The producer may need to contact the manufacturer of the salt to get a statement of what is in the salt, or may be required to locate a salt that is free of whiteners and anti-caking and flow agents. There is one exception to this rule, salt formulated with magnesium carbonate is allowed in products labeled as “made with organic ingredients.” However, using salt formulated with magnesium carbonate is prohibited in products labeled “organic” (products containing at least 95% organic ingredients) or “100% organic”. It is possible that salt could be formulated with an anti-caking or flow agent not on the list above. -

Turning Water Into Ink Student Worksheet
Turning Water into Ink Student Worksheet Name______________________________________________________________________ Overview You will look like a true magician as you pour two clear solutions together and make something that looks like real ink! What to Learn By the end of this experiment you will understand how to make a saturated solution, and how to identify a chemical reaction. Materials sodium ferrocyanide (MSDS) ferric ammonium sulfate (MSDS) 2 test tubes distilled water goggles and gloves water Lab Time CAUTION: Do not mix sodium ferrocyanide with any other chemical other than specified here, as it can produce hydrogen cyanide gas, which is lethal. Handle this chemical with care, wear gloves, and keep it locked away when not in use. 1. Measure out a tiny bit of sodium ferrocyanide into a test tube filled partway with water. You want to add enough of the crystals so that when you shake the solution (with the cap on), all of the crystals dissolve into the water and make a saturated solution. 2. Into a second test tube, dissolve another tiny bit of ferric ammonium sulfate in water, adding just enough to make a saturated solution. * 3. When you’re ready, pour one test tube into the other and note the change! 4. When finished, rinse with plenty of water and make sure to clean up lab area thoroughly. *NOTE: You can use this as real ink by using it BEFORE you combine them together like this: dip a toothpick into the first solution (sodium ferrocyanide solution) and with the tip write onto a sheet of paper. While the writing is drying, dip a piece of paper towel in the other solution (ferric ammonium sulfate solution) and gently blot along where you wrote on the paper… and the color appears as blue ink. -

EPA Source Water Protection Practices Bulletins
United States Office of Water EPA 816-F-02-019 Environmental Protection (4606) August 2002 Agency Source Water Protection Practices Bulletin Managing Highway Deicing to Prevent Contamination of Drinking Water We depend on clear roads and highways for safe travel and the continual flow of goods and services. Deicing chemicals are used to clear roads covered by snow and ice during winter weather. The runoff associated with highway deicing may contain various chemicals and sediment which have the potential to enter surface and ground water sources. This bulletin focuses on the management of highway deicing chemicals. See the bulletin on storm water runoff for additional management measures. USE OF HIGHWAY DEICING CHEMICALS Each winter, state, county, and local transportation departments stock their arsenal with the tools necessary to face whatever winter storms may bring. This arsenal includes a variety of chemicals to melt snow and ice. This preparedness has a high price tag; in the U.S., an estimated $2 billion is spent each year on chemicals, materials, labor, and equipment for winter road maintenance. The most commonly used and economical deicer is sodium chloride, better known as salt;15 million tons of deicing salt are used in the U.S. each year. Salt is effective because it lowers the freezing point of water, preventing ice and snow from bonding to the pavement and allowing easy removal by plows. However, the use of salt is not without problems. Salt contributes to the corrosion of vehicles and infrastructure, and can damage water bodies, ground water, and roadside vegetation. These issues have led to the investigation and use of other chemicals as substitutes for and supplements to salt. -

Phenethyl Isothiocyanate (Peitc) Decreases Specificity
PHENETHYL ISOTHIOCYANATE (PEITC) DECREASES SPECIFICITY PROTEIN (SP) TRANSCRIPTION FACTORS THROUGH AN ROS- DEPENDENT MECHANISM A Thesis by AARON SHANE GUTHRIE Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, Stephen Safe Committee Members, Robert Burghardt Xiuren Zhang Head of Department, Gregory D. Reinhart December 2012 Major Subject: Biochemistry Copyright 2012 Aaron Shane Guthrie ABSTRACT Isothiocyanates (ITCs) are phytochemicals highly expressed in cruciferous vegetables and these compounds are associated with the decreased incidence of cancers in populations consuming high levels of cruciferous vegetables. Several individual ITCs including phenethyl isothiocyanate (PEITC) inhibit tumor growth and angiogenesis and their anticancer activity has been linked to inhibition of cancer cell growth, survival and inflammation (NFB). It has also been demonstrated that PEITC induces reactive oxygen species (ROS) and that ROS is largely responsible for PEITC-induced cell death. To confirm PEITC-induced cancer cell death we have investigated the mechanism of action of PEITC in pancreatic cancer cell lines and PEITC induces ROS and inhibits growth and induces apoptosis (PARP cleavage). In addition, PEITC downregulates expression of several gene products including vascular endothelial growth factor (VEGF), cyclin D1 (CD1), Bcl2 and survivin and these have previously been reported in other studies. However, since these gene products are all regulated by specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4, which are overexpressed in cancer cells and tumors, we investigated the effects of PEITC on Sp proteins and observed that PEITC decreased expression of Sp1, Sp3 and Sp4 in pancreatic cancer cells.