Management of Infertility Appendixes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Assisted Hatching on Pregnancy Outcomes: a Systematic Review and Meta- Analysis of Randomized Controlled Trials
Effect of assisted hatching on pregnancy outcomes: a systematic review and meta- analysis of randomized controlled trials The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Li, Da, Da-Lei Yang, Jing An, Jiao Jiao, Yi-Ming Zhou, Qi-Jun Wu, and Xiu-Xia Wang. 2016. “Effect of assisted hatching on pregnancy outcomes: a systematic review and meta-analysis of randomized controlled trials.” Scientific Reports 6 (1): 31228. doi:10.1038/ srep31228. http://dx.doi.org/10.1038/srep31228. Published Version doi:10.1038/srep31228 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:29002519 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA www.nature.com/scientificreports OPEN Effect of assisted hatching on pregnancy outcomes: a systematic review and meta-analysis of Received: 04 February 2016 Accepted: 15 July 2016 randomized controlled trials Published: 09 August 2016 Da Li1, Da-Lei Yang1, Jing An1, Jiao Jiao1, Yi-Ming Zhou2, Qi-Jun Wu3 & Xiu-Xia Wang1 Emerging evidence suggests that assisted hatching (AH) techniques may improve clinical pregnancy rates, particularly in poor prognosis patients; however, there still remains considerable uncertainty. We conducted a meta-analysis to verify the effect of AH on pregnancy outcomes. We searched for related studies published in PubMed, Web of Science, and Cochrane library databases from start dates to October 10, 2015. -

Pemanfaatan Teknik Assisted Hatching Dalam Meningkatkan Implantasi Embrio
WARTAZOA Vol. 27 No. 1 Th. 2017 Hlm. 035-044 DOI: http://dx.doi.org/10.14334/wartazoa.v27i1.1412 Pemanfaatan Teknik Assisted Hatching dalam Meningkatkan Implantasi Embrio (Utilization of Assisted Hatching Techniques to Enhance Embryo Implantation) Arie Febretrisiana1 dan FA Pamungkas2 1Loka Penelitian Kambing Potong, PO Box I Sei Putih, Galang 20585, Sumatera Utara 2Pusat Penelitian dan Pengembangan Peternakan, Jl. Raya Pajajaran Kav. E-59, Bogor 16128 [email protected] (Diterima 29 September 2016 – Direvisi 12 Januari 2017 – Disetujui 27 Februari 2017) ABSTRACT Gestation is the main goal for in vitro fertilization. The embryo that has been developed outside the body will be transferred directly into uterus leading to the process of hatching, implantation, and pregnancy. However, approximately 85% of embryos that have been transferred were failed to implant and it might be caused by hatching failure. Hatching is the process of releasing embryo from zona pellucida. If this process does not occur, it will cause pregnancy failure. Assisted hatching is a mechanism that dealing with thinning, slicing or artificially making holes in the zona pellucida to improve hatching. The process can be applied both in fresh or frozen embryos. This review describes various methods in assisted hatching such as enzymatic, chemical, mechanical, and laser beam as well as their advantages and disadvantages. Generally, some researches show that the technology of assisted hatching can improve the percentage of hatching and implantation of the embryo. However, in spite of the benefits, there are such weaknesses find in the zona pellucida of the embryo that has been manipulated such as toxic hazard medium, the risk of damage to the blastomeres or monozygotic twinning. -

2021 National Formulary 3Rd Quarter Edition
2021 National Formulary 3rd Quarter Edition Last Revised: 08/18/2021 Version 2021Q3c Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) DRUGS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Drugs 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) DRUGS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Drugs 17 Fibrates 17 BIOSIMILARS 7 ACL Inhibitors 17 Other Drugs 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC DRUGS 18 ANTIBIOTICS 8 Penicillins & Cephalosporins 8 KIDNEY & URINARY / UROLOGICAL DRUGS 18 Tetracyclines 8 Benign Prostate Hyperplasia 18 Macrolides & Clindamycins 8 Urologic Drugs / Other Drugs 18 Sulfonamides, Sulfones & Ketolides 8 Erectile Dysfunction Drugs 18 Quinolones 8 Gout Drugs – Purine Inhibitors 19 Miscellaneous Antibiotics 8 Urinary Ph Modifiers 19 Potassium & Electrolytes 19 ANTI-VIRALS 9 Phosphorus/Calcium/Electrolyte Depleters 19 General Antivirals 9 HIV Antiviral Drugs 9 OSTEOPOROSIS (BONE) DRUGS 20 HIV Pre-Exposure Propylaxis Drugs 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) DRUGS 20 ANTI-INFECTIVES 10 Anti-Inflammatory Drugs (NSAIDS) 20 Anaerobic Anti-Infectives 10 COX-II Drugs 21 Antiparasitics 10 Analgesics, Narcotics (Opioids) 21 Antimalarials & Antiprotozoals 10 Analgesics, Salicylates, Non-Salicylates, Other 21 Antihelmintic Drugs 10 CENTRAL NERVOUS SYSTEM DRUGS 22 ANTIEMETICS 10 Anti-Anxiety Drugs (Benzodiazepines) 22 Sedative/Sleeping Drugs 22 NEUROLOGIC DRUGS 11 A.D.D. -

LHRH) Antagonist Cetrorelix and LHRH Agonist Triptorelin on the Gene Expression of Pituitary LHRH Receptors in Rats
Comparison of mechanisms of action of luteinizing hormone-releasing hormone (LHRH) antagonist cetrorelix and LHRH agonist triptorelin on the gene expression of pituitary LHRH receptors in rats Magdolna Kovacs*†‡ and Andrew V. Schally*†§ *Endocrine, Polypeptide, and Cancer Institute, Veterans Affairs Medical Center, New Orleans, LA 70112; and †Section of Experimental Medicine, Department of Medicine, Tulane University School of Medicine, New Orleans, LA 70112 Contributed by Andrew V. Schally, August 21, 2001 The mechanisms through which luteinizing hormone (LH)-releasing however, are different. LHRH agonists achieve the inhibition of hormone (LHRH) antagonists suppress pituitary gonadotroph func- gonadotropin secretion after a period of continuous exposure (1, tions and LHRH-receptor (LHRH-R) expression are incompletely un- 2, 11–14). In contrast, antagonists of LHRH produce a compet- derstood. Consequently, we investigated the direct effect of LHRH itive blockade of LHRH-R and cause an immediate cessation of antagonist cetrorelix in vitro on the expression of the pituitary the release of gonadotropins and sex steroids, reducing the time LHRH-R gene and its ability to counteract the exogenous LHRH and of the onset of therapeutic effects as compared with the agonists the agonist triptorelin in the regulation of this gene. We also com- (1, 2, 15–17). LHRH agonists such as triptorelin, leuprolide, pared the effects of chronic administration of cetrorelix and triptore- buserelin, or goserelin (1, 2, 14) have been used worldwide for lin on the LHRH-R mRNA level and gonadotropin secretion in ovari- nearly two decades, but LHRH antagonists such as cetrorelix, ectomized (OVX) and normal female rats. The exposure of pituitary ganirelix, and Abarelix have been introduced into the clinical cells in vitro to 3-min pulses of 1 nM LHRH or 0.1 nM triptorelin for 5 h practice relatively recently (1, 2, 15, 16). -

Personalized ADT
Personalized ADT Thomas Keane MD Conflicts • Ferring • Tolemar • Bayer • Astellas • myriad Personalized ADT for the Specific Paent • Cardiac • OBesity and testosterone • Fsh • High volume metastac disease • Docetaxol • Significant LUTS Cardiovascular risk profile and ADT Is there a difference? Degarelix Belongs to a class of synthe@c drug, GnRH antagonist (Blocker) GnRH pGlu His Trp Ser Tyr Gly Leu Arg Pro Gly NH2 Leuprolide D-Leu NEt Goserelin D-Ser NH2 LHRH agonists Triptorelin D-Trp NH2 Buserelin D-Ser NEt Degarelix D-NaI D-Cpa D-PaI Aph D-Aph D-Ala NH2 N-Me ABarelix D-NaI D-Cpa D-PaI D-Asn Lys D-Ala NH2 Tyr GnRH antagonists Cetrorelix D-NaI D-Cpa D-PaI D-Cit D-Ala NH2 Ganirelix D-NaI D-CPa D-PaI D-hArg D-hArg D-Ala NH2 Millar RP, et al. Endocr Rev 2004;25:235–75 Most acute CVD events are caused By rupture of a vulnerable atherosclero@c plaque The vulnerable plaque – thin cap with inflammaon Inflammation Plaque instability is at the heart of cardiovascular disease Stable plaque Vulnerable plaque Lumen Lumen Lipid core Lipid core FiBrous cap FiBrous cap Thick Cap Thin Rich in SMC and matrix Composion Rich in inflammatory cells: proteoly@c ac@vity Poor Lipid Rich Inflammatory Inflammatory state Highly inflammatory LiBBy P. Circulaon 1995;91:2844-2850 Incidence of Both prostate cancer and CV events is highest in older men Prostate cancer CV events 3500 3500 Prostate cancer All CV disease Major CV events 3000 3000 2827.1 2500 2500 2338.9 2000 2000 1719.7 1500 1500 1152.6 1008.7 1038.7 1000 1000 641.2 545.2 571.1 Age-specific incidence per 100,000 person-years 500 500 246.9 133.7 4.3 0 0 40-49 50-59 60-69 70-79 80-89 90-99 40-49 50-59 60-69 70-79 80-89 90-99 CV, cardiovascular Major CV events = myocardial infarc@on, stroke, or death due to CV disease All CV disease = major CV events + self-reported angina or revascularisaon procedures Driver, et al. -
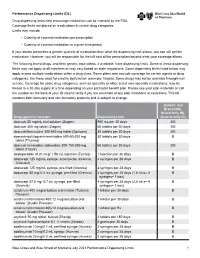
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Degarelix for Treating Advanced Hormone- Dependent Prostate Cancer
CONFIDENTIAL UNTIL PUBLISHED NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Final appraisal determination Degarelix for treating advanced hormone- dependent prostate cancer This guidance was developed using the single technology appraisal (STA) process 1 Guidance 1.1 Degarelix is recommended as an option for treating advanced hormone-dependent prostate cancer, only in adults with spinal metastases who present with signs or symptoms of spinal cord compression. 1.2 People currently receiving treatment initiated within the NHS with degarelix that is not recommended for them by NICE in this guidance should be able to continue treatment until they and their NHS clinician consider it appropriate to stop. 2 The technology 2.1 Degarelix (Firmagon, Ferring Pharmaceuticals) is a selective gonadotrophin-releasing hormone antagonist that reduces the release of gonadotrophins by the pituitary, which in turn reduces the secretion of testosterone by the testes. Gonadotrophin- releasing hormone is also known as luteinising hormone-releasing hormone (LHRH). Because gonadotrophin-releasing hormone antagonists do not produce a rise in hormone levels at the start of treatment, there is no initial testosterone surge or tumour stimulation, and therefore no potential for symptomatic flares. National Institute for Health and Care Excellence Page 1 of 71 Final appraisal determination – Degarelix for treating advanced hormone-dependent prostate cancer Issue date: April 2014 CONFIDENTIAL UNTIL PUBLISHED Degarelix has a UK marketing authorisation for the ‘treatment of adult male patients with advanced hormone-dependent prostate cancer’. It is administered as a subcutaneous injection. 2.2 The most common adverse reactions with degarelix are related to the effects of testosterone suppression, including hot flushes and weight increase, or injection site reactions (such as pain and erythema). -

Is It Time to Abandon Embryo Byopsy Techniques?”
FACULTY OF SCIENCES DEGREE IN BIOLOGY FINAL PROJECT ACADEMIC YEAR (2019-2020) TITLE: EMBRYONIC ANEUPLOIDY DETECTION BY NON-INVASIVE METHODS, A REVIEW. “IS IT TIME TO ABANDON EMBRYO BYOPSY TECHNIQUES?” AUTHOR: ÁNGEL MÁÑEZ GRAU 2 SUMMARY: Assisted reproduction is the group of techniques that help people to deal with fertility and sterility problems. These techniques have the goal of achieving a live birth, in which a healthy baby is born. To ensure that the baby is healthy and to avoid any type of pregnancy or birth defects or problems, a genetic test can be performed to the embryo, prior to the transfer and implantation in the mother. This is called the preimplantation genetic test (PGT). The test can be done to detect structural rearrangements (SR), monogenic diseases (M) or aneuploidies (A), and it is mostly done by performing an embryo biopsy on the 5th day of development, following by different genetic approaches. This technique generates lots of controversy, due to ethical and embryo viability implications, because it is not known if it significantly affects the embryo. So, to avoid this kind of problems, non-invasive techniques (niPGT) have been developed. The niPGT relies on the presence of genetic material in the embryo spent culture medium, which is analysed instead of the embryo trophectodermal cells. This techniques has several advantages over the invasive ones. The clearest one is that there is no need to harm the embryo with lasers or pipettes. The need of experimented technicians and expensive laboratory equipment are not required for this type of technique, so it makes it easier and cheaper. -

Gonadotropin Therapy in Assisted Reproduction: an Evolutionary Perspective from Biologics to Biotech
REVIEW Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech Roge´rio de Barros F. Lea˜ o, Sandro C. Esteves Andrology & Human Reproduction Clinic (ANDROFERT), Referral Center for Male Reproduction, Campinas/SP, Brazil. Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine- derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity. KEYWORDS: Gonadotropins; Ovulation Induction; Assisted Reproductive Techniques; Systematic Review. -

Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log
Clinical Policy: Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The following are gonadotropins requiring prior authorization: Menotropins (Menopur®), Follitropin alpha, recombinant (Gonal-F® RFF), Follitropin beta, recombinant (Follistim®-AQ), Urofollitropin (Bravelle®), Choriogonadotropin alfa (Ovidrel®), Human chorionic gonadotropin (Novarel®, Pregnyl®), Ganirelex acetate, Cetrorelix (Cetrotide®). FDA approved indication Menopur is indicated for development of multiple follicles and pregnancy in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Gonal-F RFF is indicated: • For induction of ovulation and pregnancy in oligo-anovulatory women in whom the cause of infertility is functional and not due to primary ovarian failure. • For development of multiple follicles in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Follistim AQ is indicated: • In women for: Induction of ovulation and pregnancy in anovulatory infertile women in whom the cause of infertility is functional and not due to primary ovarian failure. • In women for: Pregnancy in normal ovulatory women undergoing controlled ovarian stimulation as part of an In Vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI) cycle. • In men for: Induction of spermatogenesis in men with primary and secondary hypogonadotropic hypogonadism (HH) in whom the cause of infertility is not due to primary testicular failure. Bravelle is indicated: • For induction of ovulation in women who have previously received pituitary suppression. • For development of multiple follicles as part of an Assisted Reproductive Technology (ART) cycle in ovulatory women who have previously received pituitary suppression. -

5.01.610 Pharmacologic Treatment of Infertility
PHARMACY / MEDICAL POLICY – 5.01.610 Pharmacologic Treatment of Infertility Effective Date: Feb. 1, 2021 RELATED MEDICAL POLICIES: Last Revised: Jan. 21, 2021 4.02.503 Infertility and Reproductive Services Replaces: N/A Select a hyperlink below to be directed to that section. POLICY CRITERIA | DOCUMENTATION REQUIREMENTS | CODING RELATED INFORMATION | EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction Infertility is a problem or problems with the reproductive system that affects the ability to conceive. Different types of reproductive problems affect men and women, but the end result is the inability to conceive or complete a pregnancy. There are many reasons for infertility and drug options vary depending on the cause of infertility and type of infertility treatment required. Even though drug treatment exists, it does not mean it is covered; the member’s contract determines this. This policy describes when infertility drugs may be considered medically necessary if covered by the member’s contract. Note: The Introduction section is for your general knowledge and is not to be taken as policy coverage criteria. The rest of the policy uses specific words and concepts familiar to medical professionals. It is intended for providers. A provider can be a person, such as a doctor, nurse, psychologist, or dentist. A provider also can be a place where medical care is given, like a hospital, clinic, or lab. This policy informs them about when a service may be covered. Policy Coverage -

Degarelix Acetate (Firmagon) Reference Number: PA.CP.PHAR.170 Effective Date: 01/18 Last Review Date: 10/18 Revision Log
Clinical Policy: Degarelix Acetate (Firmagon) Reference Number: PA.CP.PHAR.170 Effective Date: 01/18 Last Review Date: 10/18 Revision Log Description The intent of the criteria is to ensure that patients follow selection elements established by Pennsylvania Health and Wellness® clinical policy for the use of Degarelix Acetate (Firmagon®). FDA Approved Indication(s) Firmagon is indicated for treatment of advanced prostate cancer. Policy/Criteria It is the policy of Pennsylvania Health and Wellness that Firmagon is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Prostate Cancer (must meet all): 1. Diagnosis of prostate cancer; 2. Prescribed by or in consultation with an oncologist; 3. Request meets one of the following (a, b or c): a. Starting dose does not exceed 240 mg given as two injections of 120 mg each; b. Maintenance dose does not exceed 80 mg as a single injection per 28 days; c. Dose is supported by practice guidelines or peer-reviewed literature for the relevant off- label use (prescriber must submit supporting evidence). Approval duration: 12 months B. Other diagnoses/indications: Refer to PA.CP.PMN.53 1. The following NCCN recommended uses for Firmagon, meeting NCCN categories 1, 2a, or 2b, are approved per the PA.CP.PMN.53: II. Continued Approval A. Prostate Cancer (must meet all): 1. Currently receiving medication via Pennsylvania Health and Wellness benefit or member has previously met approval criteria or the Continuity of Care policy (PA.LTSS.PHAR.01) applies; 2. Member is responding positively to therapy; 3. If request is for a dose increase, request meets one of the following: a.