The Surprising Reach of FDA Regulation of Cannabis, Even After Descheduling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Experiences of Disengagement from Mental Health Services: an Interpretative Study
EXPERIENCES OF DISENGAGEMENT FROM MENTAL HEALTH SERVICES: AN INTERPRETATIVE STUDY by CHRISTOPHER WAGSTAFF A thesis submitted to the University of Birmingham for the degree of DOCTOR OF PHILOSOPHY Nursing, Health & Population Sciences College of Medical & Dental Sciences University of Birmingham 2015 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Whilst there is evidence of a range of effective treatments available for people with severe mental health problems (SMHP), people frequently disengage from mental health services (MHS). This thesis investigates experiences of disengagement of people with SMHP and comprises two studies: 1) semi-structured interviews to elicit the experiences of people with SMHP and a history of disengagement from MHS; and 2) building on those findings, focus groups with staff from assertive outreach teams (specialising in providing care for people with SMHP and a history of disengagement). The participants were those perceived as the most disengaged from MHS yet they were willing to engage with the research. Interpretative phenomenological analysis was used to develop themes for individual participants and then across the participants. Disengagement from MHS was part of a wider experience of a limited connection to social structures, including an ambivalent and complex relationship with MHS. -

Hallucinogens - LSD, Peyote, Psilocybin, and PCP
Hallucinogens - LSD, Peyote, Psilocybin, and PCP Hallucinogenic compounds found in some • Psilocybin (4-phosphoryloxy-N,N- plants and mushrooms (or their extracts) dimethyltryptamine) is obtained from have been used—mostly during religious certain types of mushrooms that are rituals—for centuries. Almost all indigenous to tropical and subtropical hallucinogens contain nitrogen and are regions of South America, Mexico, and classified as alkaloids. Many hallucinogens the United States. These mushrooms have chemical structures similar to those of typically contain less than 0.5 percent natural neurotransmitters (e.g., psilocybin plus trace amounts of acetylcholine-, serotonin-, or catecholamine- psilocin, another hallucinogenic like). While the exact mechanisms by which substance. hallucinogens exert their effects remain • PCP (phencyclidine) was developed in unclear, research suggests that these drugs the 1950s as an intravenous anesthetic. work, at least partially, by temporarily Its use has since been discontinued due interfering with neurotransmitter action or to serious adverse effects. by binding to their receptor sites. This DrugFacts will discuss four common types of How Are Hallucinogens Abused? hallucinogens: The very same characteristics that led to • LSD (d-lysergic acid diethylamide) is the incorporation of hallucinogens into one of the most potent mood-changing ritualistic or spiritual traditions have also chemicals. It was discovered in 1938 led to their propagation as drugs of abuse. and is manufactured from lysergic acid, Importantly, and unlike most other drugs, which is found in ergot, a fungus that the effects of hallucinogens are highly grows on rye and other grains. variable and unreliable, producing different • Peyote is a small, spineless cactus in effects in different people at different times. -

Cannabis – a Complex and Rapidly Evolving Landscape
CANNABIS – A COMPLEX AND RAPIDLY EVOLVING LANDSCAPE Abstract ABOUT THE AUTHOR The humble Cannabis sativa plant, cultivated for millennia for its psychoactive properties and more, is today considered one of the most controversial and complex plants in the world. Starting in the early to mid-20th century, much of its use became recreational, but by the early 1970s discoveries began to emerge around its potential medical efficacies. This article will discuss current knowledge of how cannabis engages with the brain and the endocannabinoid system (ECS) and provide an overview of the new market landscapes brought about by changes in governing laws Dr. Georgiana Willwerth-Pascutiu and regulations, which are affecting usage by our current and potential [email protected] customers. It will also explore the additional hazards, concerns, and Georgiana Willwerth-Pascutiu is Vice President, Global Medical Director considerations of cannabis use in countries where it remains illegal. at RGA. She is board certified in Insurance Medicine by the American Introduction Academy of Insurance Medicine (AAIM) and specialized in internal medicine, Naturally occurring psychoactive substances have been part of human life nephrology and ultrasonography. for millennia. One of the most frequently utilized plant sources of these Dr. Willwerth-Pascutiu is also a past substances, Cannabis sativa, is also the best-known worldwide. For the president of the Canadian Life Insurance Medical Officers Association (CLIMOA) past half-century, scientific and medical interest in its many compounds, and currently chairs its scientific known as cannabinoids, has been increasing. Today, the two best-known, committee. She is a frequent presenter and has contributed several articles to delta-9 tetrahydrocannabinol (THC), its psychoactive chemical, and insurance industry publications. -

Appendix 1 (As Supplied by the Authors)
Appendix 1 (as supplied by the authors): Sources of information regarding how each Canadian province and territory is allowing cannabis retail and online sales, and store locations Jurisdiction Source Store locations Provinces Newfoundland and Labrador Legislation: Bill 20: An Act Clarenville Green Stop (Esso), Clarenville, 258 Memorial Drive, Respecting the Control and Sale A5A1N9 of Cannabis1 C-Shop, Bay Roberts, 230 Conception Bay Highway, A0A1G0 Regulations: Newfoundland and C-Shop, Carbonear, 120 Columbus Drive, A1Y1B3 Labrador Cannabis Regulations2 C-Shop, Conception Bay South, 166 Conception Bay Highway, A1W3A6 List of Retail Stores: Store C-Shop, Corner Brook, 5 Murphy Square, A2H1R4 Locator (Cannabis NL)3 C-Shop, Gander, 100 Laurell Road, A1V2V5 Online Store: Cannabis NL4 C-Shop, Grand Falls-Windsor, 17 Cromer Ave, A2A1X3 C-Shop, Mount Pearl, 150 Old Placentia Road, A1N4Y9 C-shop, St. John's, 260 Blackmarsh Road, A1E1T2 C-shop, St. John's, 55 Stavanger Drive, A1A5E8 C-Shop, Stephenville, 62 Prince Rupert Drive, A2N3W7 Deer Lake Green Stop, Deer Lake, 31 Upper Nicholsville Rd, A8A2G1 High North, Labrador City, 1 Neal Drive, A2V1Y5 Miawpukek Cannabis Boutique, Conne River, 19 Miawpukek Drive, A0H1J0 Paradise Green Stop, Paradise, 1316 Topsail Rd, A1L1N9 The Herbal Centre, St. John's, 394 Kenmount Road, A1B3R2 The Natural Vibe, St. John's, 306 Water Street, A1C1B8 The Reef Cannabis Shop, Holyrood, 386 CBS Highway, A0A2R0 Thomas H. Clarke's Distribution, Portugal Cove - St. Phillips, 1614 Portugal Cove Road, A1M3G3 Tweed, Conception Bay, 81 Conception Bay Highway S Unit 3, A1W3A3 Tweed, Corner Brook, 62 Broadway Avenue, A2H6H4 Tweed, Happy Valley-Goose Bay, 27 Aspen Drive, A0P1C0 Tweed, Mount Pearl, 50 Commonwealth Ave Unit 5, A1N1X1 Tweed, St. -

Hallucinogens - LSD, Peyote, Psilocybin, and PCP
Information for Behavioral Health Providers in Primary Care Hallucinogens - LSD, Peyote, Psilocybin, and PCP What are Hallucinogens? Hallucinogenic compounds found in some plants and mushrooms (or their extracts) have been used— mostly during religious rituals—for centuries. Almost all hallucinogens contain nitrogen and are classified as alkaloids. Many hallucinogens have chemical structures similar to those of natural neurotransmitters (e.g., acetylcholine-, serotonin-, or catecholamine-like). While the exact mechanisms by which hallucinogens exert their effects remain unclear, research suggests that these drugs work, at least partially, by temporarily interfering with neurotransmitter action or by binding to their receptor sites. This InfoFacts will discuss four common types of hallucinogens: LSD (d-lysergic acid diethylamide) is one of the most potent mood-changing chemicals. It was discovered in 1938 and is manufactured from lysergic acid, which is found in ergot, a fungus that grows on rye and other grains. Peyote is a small, spineless cactus in which the principal active ingredient is mescaline. This plant has been used by natives in northern Mexico and the southwestern United States as a part of religious ceremonies. Mescaline can also be produced through chemical synthesis. Psilocybin (4-phosphoryloxy-N, N-dimethyltryptamine) is obtained from certain types of mushrooms that are indigenous to tropical and subtropical regions of South America, Mexico, and the United States. These mushrooms typically contain less than 0.5 percent psilocybin plus trace amounts of psilocin, another hallucinogenic substance. PCP (phencyclidine) was developed in the 1950s as an intravenous anesthetic. Its use has since been discontinued due to serious adverse effects. How Are Hallucinogens Abused? The very same characteristics that led to the incorporation of hallucinogens into ritualistic or spiritual traditions have also led to their propagation as drugs of abuse. -

A Review on Cannabis Sativa: Its Compounds and Their Effects
Int. J. Pharm. Sci. Rev. Res., 53(2), November - December 2018; Article No. 12, Pages: 59-63 ISSN 0976 – 044X Review Article A Review on Cannabis sativa: Its Compounds and Their Effects Ranju Rajput, *Dr. Krishan Kumar Department of Food and Biotechnology, Jayoti Vidyapeeth Women’s University, Jaipur, Rajasthan, India. *Corresponding author’s E-mail: [email protected] Received: 01-11-2018; Revised: 25-11-2018; Accepted: 10-12-2018. ABSTRACT Our society often considered the use of cannabis is an under-reported activity. Cannabis is used to relieve neuropathic and chronic pain. Cannabis, produced from the Cannabis sativa plant, have been used in three forms: herbal cannabis, the dried leaves and flowering tops The resin of the cannabis is the pressed secretions of the plant, known as ‘hashish’ or ‘charash. Cannabis sativa is an herbaceous species originated from Central Asia. It has been used in medicine and as a source of textile fiber since ancient times. The cannabis sativa is a fast growing plant attracted the people’s interest because of its multi-purpose applications. It is a rich source of photochemical, cellulose and woody fibers. The more interest is also due to its metabolites which show potent bioactivities on human health. In this review, the phytochemicals is discussed by putting a special emphasis on molecules including cannabinoids, terpenes and phenolic compounds. Cannabinoids are represented as the most studied group of compounds, because of their wide range of pharmaceutical effects in humans, including psychotropic activities. This article aims to update the current knowledge and evidence of using cannabis and its derivatives with a view to the sociolegal context and perspectives for future research. -

Rockledge City Council Regular Meeting Notice and Agenda
ROCKLEDGE CITY COUNCIL REGULAR MEETING NOTICE AND AGENDA Wednesday, February 1, 2017 - 6:00 p.m. Chairman Thomas J. Price Presiding Council Chamber, Rockledge City Hall, 1600 Huntington Lane, Rockledge, FL 32955 *~*~*~*~*~*~* EVERY PERSON ADDRESSING THE CITY COUNCIL MUST COMPLETE A SPEAKER'S CARD The cards are located near the door of the Council Chamber. Completed cards are to be given to the City Clerk before the meeting convenes or prior to the introduction of a particular agenda item. *~*~*~*~*~*~* 1. CALL TO ORDER / ROLL CALL 2. INVOCATION l Councilman Hartselle 3. SALUTE TO THE FLAG 4. APPROVAL OF MINUTES l Regular Meeting on January 18, 2017 Documents: COUNCIL MINUTES 2017 01-18.PDF 5. PRESENTATIONS A. Mayor Price 1. Certificate Of Completion To Councilman Daski: 2016 Florida League Of Cities Advanced Institute For Elected Municipal Officials B. Public Works Director Poole 1. Video: Know Your Waterways 6. FINANCIAL / BUDGET REPORT l None 7. PUBLIC HEARINGS / ORDINANCES / RESOLUTIONS A. Resolution: Providing for the Apportionment of $4.00 of the $14.00 Base Sewer Service Charge Documents: 2017- RESOLUTION SEWER CHARGE APPORTIONMENT.PDF B. Public Hearing: VE-17-01, Vacate Public Utility Easement, Lots 25, 26, 27 and 28, Angela Avenue, Casa Loma Subdivision Documents: PUBLIC HEARING NOTICE VACATE EASEMENT PORTION OF ANGELA AVE CASA LOMA SUBDIVSION.PDF C. Resolution: Vacating Public Utility Easement, Lots 25, 26, 27 and 28, Angela Avenue, Casa Loma Subdivision Documents: 2017- RESOLUTION VACATING EASEMENT, CASA LOMA SUBDIVISION BLOCK A (VE-17-01, RJM MERCO).PDF D. Ordinance: First Reading, Relating to Cannabis Dispensing Facilities and Imposing a Temporary Moratorium on the Opening of Any New Cannabis Dispensing Facility Documents: ORDINANCE NO. -

The Canadian Cannabis Story
A Generational Investment Opportunity THE CANADIAN CANNABIS STORY JOIN THE CONVERSATION / Echelon Wealth Partners echelonpartners.com TABLE OF CONTENTS 3 The Canadian Cannabis Story: A Generational Investment Opportunity 4 Cannabis: A Brief History 5 The Many Forms of Cannabis 5 An Increase in Legal Cannabis-based Products 5 Medical Use 8 Cannabis as an Opiod Alternative 11 Cannabis and Canada: A Strong Growth Story 14 A Global Cannabis Boom: The Next Stage 17 Canadian Cannabis Stocks - An Investment Opportunity to Consider 19 Endnotes echelonpartners.com 2 The Canadian Cannabis Story: A Generational Investment Opportunity THE CANADIAN CANNABIS STORY: A GENERATIONAL INVESTMENT OPPORTUNITY By Echelon Wealth Partners The Canadian Cannabis Story aims to provide readers with a comprehensive look at the cannabis market in Canada through its history, growth, and various production sectors to illuminate the investment opportunity this sector will afford in a rapidly growing global market. The increasing trend in cannabis decriminalization and legalization, both in North America and around the world, has awakened the interest of the investment community. The unique and potential medicinal properties of cannabis and its versatility for other commercial uses are considered the harbingers of an investment with significant growth potential. Canada legalized marijuana for recreational use on October 17, 2018 echelonpartners.com 3 The Canadian Cannabis Story: A Generational Investment Opportunity CANNABIS A Brief History Marijuana is produced from the flower and leaves of cannabis plants, which grow naturally in humid temperate conditions on all continents.1 The two most important varieties of the cannabis plant are sativa and indica, with hemp being a specific species of the sativa plant. -
HPLC Application Note Cannabinoids
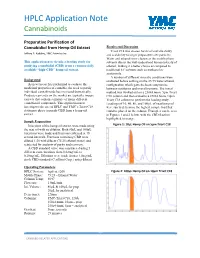
HPLC Application Note Cannabinoids Preparative Purification of Results and Discussion Cannabidiol from Hemp Oil Extract Triart C18 was chosen for its overall durability Jeffrey A. Kakaley, YMC America Inc. and scalability to larger preparative size particles. Water and ethanol were chosen as the mobile phase This application note details a loading study for solvents due to the well-understood human toxicity of purifying cannabidiol (CBD) from a commercially ethanol, making it a better choice as compared to available “high-CBD” hemp oil extract. traditional LC solvents such as methanol or acetonitrile. A number of different isocratic conditions were Background evaluated before settling on the 25:75 water:ethanol As new research is performed to evaluate the configuration which gave the best compromise medicinal properties of cannabis, the need to purify between resolution and overall runtime. The initial individual cannabinoids has increased dramatically. method was worked out on a 250x4.6mm, 5µm Triart Products currently on the market are typically impure C18 column and then scaled to a 250x4.6mm, 10µm extracts that contain a mixture of many different Triart C18 column to perform the loading study. cannabinoid compounds. This application note Loadings of 10, 40, 80, and 100µL of neat hemp oil investigates the use of HPLC and YMC’s Triart C18 were run to determine the highest sample load that stationary phase to purify CBD from a hemp oil could be placed on the column. Examples can be seen extract. in Figures 1 and 2 below, with the CBD fraction highlighted in orange: Sample Preparation Figure 1: 10µL Hemp Oil on 5µm Triart C18 Injections of the hemp oil extract were made using the neat oil with no dilution. -
Cannabinoid Profile and Elemental Uptake of Cannabis Sativa L. As ¯ ¯ 1 Influenced by Soil Characterxst,Cs
Cannabinoid Profile and Elemental Uptake of Cannabis sativa L. as ¯ ¯ 1 Influenced by Soil Characterxst,cs C.~ B. Coffman and W. A. Gentner ABSTRACT The consumption of Cannabis products (marihuana) derived from domestic and foreign sources persists in the United States despite its illegality and health hazards. The objectives of this investigation were: 1) to evaluate relationships between soil and plant elements, cannabi- noids, and growth of Cannabis sativa L., and 2) to evaluate the practicality of using chemical analysis of Cannabis products to determine their geographic origin. Knowledge of geographic origin is useful to governmental agencies investigating illicit narcotic traffic. Cannabis sativa L. was grown on 11 different soils for 45 days in the greenhouse. Soils differed significantly in 15 measured elements and pH. Plants were grown from seed of Afghan origin. The following cannabinoids were extracted and measured from leaf tissue: cannabicyclol (CCC), cannabidiol (CBD), Ae.Tetrahydrocannabinol (A~THC), and cannabinol (CBN). Fifteen elements measured in leaf tissue and correlated with soil and cannabinoid measurements. Soil pH was negatively cor- related with leaf concentrations of Mn, Fe, Zn, and S. Extractable soil Mg was negatively correlated with N, A°THC and CBD concentrations in leaf tissue (p 0.05). Plant height was negatively correlated with A°THC concentration, suggesting enhancement of the narcotic principle of marihuana when grown under stress. Ex- tractable soil P205 was negatively correlated with CBD concentration while extractable soil Zn was positively cor- related with CCCconcentration. Several correlations be- tween soil and plant characteristics having potential value for determination of geographic origin of marihuana were elucidated. -

The Determination of CBD and General Cannabinoid Content In
No. SSI-HPLC-018 High Performance Liquid Chromatography The Determination of CBD and General Cannabinoid Content in Hemp Oils Using HPLC with UV Detection No. HPLC-018 ■ Introduction Medical marijuana generally possesses high levels of CBD oil is derived as concentrate from CO2 or the therapeutic cannabidiol, CBD, and lower levels butane extraction of hemp, sometimes followed by (generally less than 0.3%) of the psychotropic steam distillation or ethanol distillation for tetrahydrocannabinol, d9-THC. Pain mitigation and purification. The Farm Bill of 2014 distinguishes reduced severity of nausea and seizures are just a hemp from marijuana, yet interpreting the law is few of the therapeutic benefits reported by medical difficult in that “CBD oil” may be classified as cannabis patients. Little has been done to better marijuana. understand the chemistry of benefits from CBD. To complicate matters, there is evidence that a The FDA has issued warning letters to firms that combination of CBD, a host of other minor market unapproved new drugs allegedly contain cannabinoids and a complex array of terpenoids may CBD. As part of these actions, the FDA has be the most beneficial – called the “entourage determined the cannabinoid content of some hemp effect.” CBD-rich oil has become increasingly products and many were found to contain levels of popular and is administered via sublingual drops, gel CBD that are very different from the label claim. It is capsules or as a topical ointment. important to note that such products are not approved by the FDA for the diagnosis, cure, The main source of CBD-rich oil is industrial hemp. -

The Organic Chemistry of Drug Synthesis
The Organic Chemistry of Drug Synthesis VOLUME 2 DANIEL LEDNICER Mead Johnson and Company Evansville, Indiana LESTER A. MITSCHER The University of Kansas School of Pharmacy Department of Medicinal Chemistry Lawrence, Kansas A WILEY-INTERSCIENCE PUBLICATION JOHN WILEY AND SONS, New York • Chichester • Brisbane • Toronto Copyright © 1980 by John Wiley & Sons, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department, John Wiley & Sons, Inc. Library of Congress Cataloging in Publication Data: Lednicer, Daniel, 1929- The organic chemistry of drug synthesis. "A Wiley-lnterscience publication." 1. Chemistry, Medical and pharmaceutical. 2. Drugs. 3. Chemistry, Organic. I. Mitscher, Lester A., joint author. II. Title. RS421 .L423 615M 91 76-28387 ISBN 0-471-04392-3 Printed in the United States of America 10 987654321 It is our pleasure again to dedicate a book to our helpmeets: Beryle and Betty. "Has it ever occurred to you that medicinal chemists are just like compulsive gamblers: the next compound will be the real winner." R. L. Clark at the 16th National Medicinal Chemistry Symposium, June, 1978. vii Preface The reception accorded "Organic Chemistry of Drug Synthesis11 seems to us to indicate widespread interest in the organic chemistry involved in the search for new pharmaceutical agents. We are only too aware of the fact that the book deals with a limited segment of the field; the earlier volume cannot be considered either comprehensive or completely up to date.