A Sheffield Hallam University Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Local Environment Agency Plan
EA-NORTH EAST LEAPs local environment agency plan SOUTH YORKSHIRE AND NORTH EAST DERBYSHIRE CONSULTATION REPORT AUGUST 1997 BEVERLEY LEEDS HULL V WAKEFIELD ■ E n v ir o n m e n t A g e n c y Information Services Unit Please return or renew this item by the due date Due Date E n v ir o n m e n t A g e n c y YOUR VIEW S Welcome to the Consultation Report for the South Yorkshire and North East Derbyshire area which is the Agency's view of the state of the environment and the issues that we believe need to be addressed during the next five years. We should like to hear your views: • Have we identified all the major issues? • Have we identified realistic proposals for action? • Do you have any comments to make regarding the plan in general? During the consultation period for this report the Agency would be pleased to receive any comments in writing to: The Environment Planner South Yorkshire and North East Derbyshire LEAP The Environment Agency Olympia House Gelderd Road Leeds LSI 2 6DD All comments must be received by 31st December 1997. All comments received on the Consultation Report will be considered in preparing the next phase, the Action Plan. This Action Plan will focus on updating Section 4 of this Consultation Report by turning the proposals into actions with timescales and costs where appropriate. All written responses will be considered to be in the public domain unless consultees explicitly request otherwise. Note: Whilst every effort has been made to ensure the accuracy of information in this report it may contain some errors or omissions which we shall be pleased to note. -

Policing-Policy-During-Strike-Report
' The Police Committee Special Sub-Committee at their meeting on 24 January 19.85 approved this report and recommended that it should be presented to the Police Committee for their approval. In doing so, they wish to place on record their appreciation and gratitude to all the members of the County Council's Department of Administration who have assisted and advised the Sub-Committee in their inquiry or who have been involved in the preparation of this report, in particular Anne Conaty (Assistant Solicitor), Len Cooksey (Committee Administrator), Elizabeth Griffiths (Secretary to the Deputy County Clerk) and David Hainsworth (Deputy County Clerk). (Councillor Dawson reserved his position on the report and the Sub-Committee agreed to consider a minority report from him). ----------------------- ~~- -1- • Frontispiece "There were many lessons to be learned from the steel strike and from the Police point of view the most valuable lesson was that to be derived from maintaining traditional Police methods of being firm but fair and resorting to minimum force by way of bodily contact and avoiding the use of weapons. My feelings on Police strategy in industrial disputes and also those of one of my predecessors, Sir Philip Knights, are encapsulated in our replies to questions asked of us when we appeared before the House of Commons Select Committee on Employment on Wednesday 27 February 1980. I said 'I would hope that despite all the problems that we have you will still allow us to have our discretion and you will not move towards the Army, CRS-type policing, or anything like that. -

Derbyshire. Brampton
DIRECTORY.] DERBYSHIRE. BRAMPTON. 51 Decorated: the exterior walls exhibit various singular stone Sitwell hart. J.P. of Renishaw Hall, Eckington, Messrs. Wil figures of Early English date, including effigies under cano- cockson Brothers and John Brown esq. are the principal pies of SS. Peter and Paul: the north aisle retains a piscina landowners. The soil is light clay; subsoil, clay. The parish with a trefoiled head: the chancel has a flat Perpendicular is chiefly agricultural ; a small portion of it is woodland. roof, the east window and two others on the south being of The acreage is 7,915; rateable value, £19,549; the popula the same period: in the wall at the west end of the nave is tion in 1881 was 6,385. a stone slab with inscription, in Lombardic characters, to PosT OFFICE.-John Collis, receiver. Letters from Chester- Matilda le Caus, probably the heiress of that barony, who field at; 8.30 a.m.; dispatched at 6 p.m. The nearest died in 1224 : through a quatrefoil opening at its upper end telegraph & money order office is at New Brampton appear the head and shoulders of a female in has-relief, and a narrow oblong opening at the bottom discloses the feet and PosT & M. 0. 0., S. B. & Annuity & Insurance Office, Cut- lower part of the drapery: there are monuments of the 17th thorpe.-Clemep.t Needham, sub-postmaster. Letters, and 18th centuries to the Clarkes of Somersall, and beneath via Chesterfield, received at 7·30 a.m. ; dispatched at 5·25 one of these a small brass to Nicholas Clarke of Brampton, p.m.; no delivery or dispatch on sunday. -

JNC Thermodynamic Database for Performance Assessment of High-Level Radioactive Waste Disposal System
JNC TN8400 99-070 JP0055253 JNC Thermodynamic Database for Performance Assessment of High-level Radioactive Waste Disposal System November, 1999 330030611® Tokai Works Japan Nuclear Cycle Development Institute JL- 1 / A s • mm T3i9-i 194 Inquiries about copyright and reproduction should be addressed to: Technical Information Section, Administration Division, Tokai Works, Japan Nuclear Cycle Development Institute 4-33 Muramatsu, Tokai-mura, Naka-gun, Ibaraki-ken, 319-1194 Japan © 1&W&-*)-1 9 ;u|i8§$8$f (Japan Nuclear Cycle Development Institute) 1999 Please be aware that all of the Missing Pages in this document were originally blank pages JNC TN8400 99-070 November, 1999 JNC Thermodynamic Database for Performance Assessment of High-level Radioactive Waste Disposal System Mikazu Yui*, Jiro Azuma*, and Masahiro Shibata* Abstract This report is a summary of status, frozen datasets, and future tasks of the JNC thermodynamic database (JNC-TDB) for assessing performance of high-level radioactive waste in geological environments. The JNC-TDB development was carried out after the first progress report on geological disposal research in Japan (H3). In the development, thermodynamic data (equilibrium constants at 25 °C, 1=0) for important radioactive elements were selected/determined based on original experimental data using different models (e.g., SIT, Pitzer). As a result, the reliability and traceability of the data for most of the important elements were improved over those of the PNC-TDB used in H-3 report. For detailed information of data analysis and selections for each element, see the JNC technical reports listed in this document. *: Waste Isolation Research Division, Tokai Works, Japan Nuclear Cycle Development Institute (JNC) JNC TN8400 99-070 -^-^ (JNC-TDB) (m % m. -

Rotherham Local Plan
making sense of heritage Rotherham Local Plan Archaeology Scoping Study of Additional Site Allocations Ref: 79971.01 November 2013 Rotherham Local Plan, Archaeology Scoping Study of Additional Site Allocations Prepared for: Rotherham Metropolitan Borough Council, Planning Policy – Planning & Regeneration, Environment & Development Services, Riverside House, Main Street, Rotherham, S60 1AE. Prepared by: Wessex Archaeology, Unit R6 Riverside Block, Sheaf Bank Business Park, Prospect Road, Sheffield, S2 3EN. www.wessexarch.co.uk November 2013 79971.01 © Wessex Archaeology Ltd 2013, all rights reserved Wessex Archaeology Ltd is a Registered Charity No. 287786 (England&Wales) and SC042630 (Scotland) Rotherham Local Plan Archaeology Scoping Study of Additional Site Allocations Quality Assurance Project Code 79971 Accession n/a Client n/a Code Ref. Planning n/a Ordnance Survey 446233, 391310 (centred) Application (OS) national grid Ref. reference (NGR) Version Status* Prepared by Checked and Approver’s Signature Date Approved By v01 E GC APN 18/11/13 File: S:\PROJECTS\79971 (Rotherham LDF 2)\Report\Working versions v02 F GC APN 28/11/13 File: S:\PROJECTS\79971 (Rotherham LDF 2)\Report v03 F AG AB 04/06/14 File: S:\PROJECTS\79971 (Rotherham LDF 2)\Report File: File: * I= Internal Draft; E= External Draft; F= Final DISCLAIMER THE MATERIAL CONTAINED IN THIS REPORT WAS DESIGNED AS AN INTEGRAL PART OF A REPORT TO AN INDIVIDUAL CLIENT AND WAS PREPARED SOLELY FOR THE BENEFIT OF THAT CLIENT. THE MATERIAL CONTAINED IN THIS REPORT DOES NOT NECESSARILY STAND ON ITS OWN AND IS NOT INTENDED TO NOR SHOULD IT BE RELIED UPON BY ANY THIRD PARTY. -

Agenda Item No: 2
AGENDA ITEM NO: 2 COMMITTEE/SUB Planning Committee DATE OF MEETING 26 th April 2004 TITLE DELEGATION PUBLICITY For Publication CONTENTS Items approved by the Group Leader, Development Management under the following Delegation references:- Building Regulations (pink sheets) P150D and P160D, P570D P580D Planning Applications (green sheets) P020D, P200D to P250D, P270D to P320D, P350D to P370D, P390D, P420D to P440D Felling and Pruning of Trees (blue sheets) P100D, P120D, P130D Agricultural and Telecommunications (yellow sheets) P330D and P340D RECOMMENDATIONS Not applicable LIST OF BACKGROUND PAPERS Relevant applications DELEGATED ITEM 1 TH BUILDING REGULATIONS DETERMINED AS AT 9 April 2004 Plan No and Ward Description, Site and Name Decision 04/00054/OTHFP Re development to provide new CON coach station facilities STLEON Bus Station And Premises New Beetwell Street Chesterfield Derbyshire S40 1QR Chesterfield Borough Council 04/00069/OTHFP Two storey extension CON BRIMN Red Lion Public House Church Street Chesterfield Derbyshire The Union Pub Co 04/00076/OTHFP Disabled lift access and internal CON alterations STLEON 87 New Square Chesterfield Derbyshire S40 1AH Chesterfield Borough Council 04/00077/MULFP Extension and internal alterations CON LOWOOD 30 Netherthorpe Close Staveley Chesterfield Derbyshire S43 3PX Mrs J Whybrow 04/00088/DOMFP Detached bungalow CON BROCKW Land To Rear Of 148 Newbold Road Newbold Chesterfield Derbyshire Mr P Dove 04/00091/DEXFP Two storey extension CON WEST 54 Cuttholme Road Loundsley Green Chesterfield Derbyshire -

NRA Dioxins and the River Doe Lea
SJfcr* U>af-<J- (^oalik Si" NRA Dioxins and the River Doe Lea Plain English Report ?"%^hitebrici fA U , 15J , / * » •*« | fJWl&rougl ^ A 671 Lodge \ K V * I \VWestfield iT^S % ' Bo (jht<Xi.fi<ilds •,^:- “ TM ■*Y-*~ 'Srkl' Pnory | ' I X r ^ . , . f y j r % v-----A- - ^ Breck. ••'. VT Fm ; ■* ■ $ 1 3 ester i a ^ __ * ^ v _ Mine I s '*,Barrow Hiui yrc "tci' (dis) I -Wr ' ; l ) r J M o o o f S r ^ , p <o 1 m l j Clowne < y /’ '•‘ .V '-H fT I^X^AtjoWne . Nethertforpe ^ X J n ?il ' ' r«/ ». V Wollingwood W >.".// x«J$®-EY ^ --y] U ^tMiddletrottjiT^^P. - /t fflaw ]w.; : ■■ ■:•$fl x Woodii^eD**^*^^J 08 ’ / 'vf $ P — SnutttuCvood Common f l i p p y \ . -fM Oxcroft i o t | s | x . I M V fEstatej/'-- /# i',V Inkersall , I __w ' he d b „ ? 0 Wi^inWfc*t Elmton p,irk "W eElmt of 153 Brimii-K^on h i%»;••' C o c u r v o a c r X i 166 ! /# / *rJ . - 1 •'fU_vW4_-/Cai Farnsworth > v % - • ■ Limekiln // Fw Field B oU over M oor ^ ,uckn?S y ^ i 4> Lorocourse SL / 407 7 BOLS0VER4 ° I / I - ^ 1!,. f e ^ M Moor________|x ^AlTi^ ^aCo1- Green Town H *5 Fo ‘ <to * /7 °T~Si/^tonWoo~d^°^-trf Sprmot /,- 94 1I \X 3Hn» ru, ,SpdngwC j m J Fm M61 E • i V Roi JUlty u 27 1 GlapweiT 1166 smoor t|VV ■ >\ 148Ji 'l (%\x\ Vy Heath' Lanes ^ *1* \ , , o , r j 5 V -'■• 1 >5A ^ C hapel/ PH 5 . -

Derbyshire Mi Ellany
DERBYSHIRE MI ELLANY lli rrtlr,:.s.o s,rn, r, JPm. n cl-n v, IDEIRttr\y s t{ The Local History Bulletin of the Derbyshire Archaeological Society Volume I Autumn lg7l Part 2 DERBY SH I RE li{l SCELLANY Vol. Vlll Pqrt 2 Autumn 1977 CONTENTS P age Early Settlement on the site of Chesterfield 39-46 by Philip Riden Gathokewell 46 by R.W.P. Cockerton The Ticknall parish documents 47-53 by J. IIYde Derbyshire Historic Buildings Trust - an appeal 53 by D.J. Latham The Darleys of Wistow and Buttercrambe 54-60 by Ernest Paulson A Frenchrnan's visit to Derbyshire in 1785 61-65 by Marian Bell From the Journal of Francois de la Rochefoucauld - 65-68 An account of a visit to Mr. Swift's mill, Derby, 1785 by E.M. BettY Hughes Two lost footpaths on Combs Edge 69 -70 by Marguerite A. Bellhouse n<, naI L - Itinerant Lecturers 'A by John Heath no n, Ice llouse at Middleton Hall, Middleton'by-Youlgreave by J.F. Marsh 74 - 75 The Aston-on-Trent tramwaY by John Heath Book notes 76 by D.V. Fowkes E DITO R Mise J. Sinar, Derbyshire Becord Office, Coubty Offices, Matlock, DE4 3AG. Editorial Assistant: D.V. Fowkes Production Assistant: V.S. Smith Distribution SecretarY: Miss J. Allen Treasurer: Mr. T. Larimore, 43 Reginald Road South, Chaddesden, Derby, DE2 6NG.. All enquiries regarding new membership and distribution should be addressed to the Treasurer. EDITOR'S NOTE The subscription to Miscellany has been held steady through many years of inflation, ttrrough the hard work of our production team headed by Victor Smith' Over this p".ioI the standard of reproduction rose appreciably, culminating in the change to offset lithography in the last issue' The combined rising cost of paper, printing and postage now make it necessary to raise the subscription-to f,I.50 yearly for members of the Derbyshire Archaeological Society and f,l.75 yearly for others' There i's not enough material in hand for the next issue. -

Local Environment Agency Plan
6 o x I local environment agency plan SOUTH YORKSHIRE & NORTH EAST DERBYSHIRE FIRST ANNUAL REVIEW May 1999 BARNSLEY ROTHERHAM SHEFFIELD CHEST ELD E n v ir o n m e n t Ag e n c y NATIONAL LIBRARY & INFORMATION SERVICE HEAD OFFICE Rio House, Waterside Drive, Aztec West. Almondsbury, Bristol BS32 4UD South Yorkshire & North East Derbyshire LEA P First Annua! Review SOUTH YORKSHIRE AND NORTH EAST DERBYSHIRE AREA ADMINISTRATIVE BOUNDARIES W . 'H D i SwllhoJ* j Oram iRNSLEY DONCASTER ) ROTHERHAM SHEFFIELD (DERBYSHIRE DALES) KEY CHESTERF.IEUD) BOLSOVER - CATCWENT BOUNDARY RIVER ADMINISTRATIVE BOUNDARY MAIN ROAD SGRTH EAST \ 0 2 4 6 8 10km ___1 i_________ i_________ i_________ i_________ i Scale ENVIRONMENT AGENCY 032505 South Yorkshire & North East Derbyshire LEAP First Annual Review EXECUTIVE SUMMARY The South Yorkshire & North East Derbyshire LEAP First Annual Review reports on the progress made during the last year against LEAP actions. The actions published in the LEAP are supplemental to our everyday work on monitoring, surveying and regulating to protect the environment. Some of the key achievements on our everyday work include: i) In September 1998 Michael Clapham MP officially opened the Bullhouse Minewater Treatment Plant. The scheme is a pioneering £1.2m partnership project funded by European Commission, Coal Authority, Environment Agency, Hepworths Building Products, Barnsley MBC and Yorkshire Water. Within one week a visible reduction could be seen in ochre levels in the River Don, after more than 100 years of pollution. ii) Monckton Coke and Chemical Company have successfully commissioned a combined heat and power plant, costing approximately £7 million. -

Frontiers in Interfacial and Nano Catalysis
Frontiers in Interfacial and Nano Catalysis Meeting of the Catalysis and Chemical Transformations Program Chemical Sciences, Geosciences and Biosciences Division Office of Basic Energy Sciences U.S Department of Energy Wintergreen, VA May 23-26, 2007 Frontiers in Interfacial and Nano Catalysis Meeting of the Catalysis and Chemical Transformations Program Chemical Sciences, Geosciences and Biosciences Division Office of Basic Energy Sciences, U.S Department of Energy Wintergreen, VA — May 23-26, 2007 Cover figures (clockwise): 1. Nuzzo, R., et al.: HRTEM of oxygen-treated Au particles with various sizes and shapes 2. Liu, M.: Potential energy profiles for the interactions of O2 and LaSrMn electrolyte 3. Stair, P.: Oxidative dehydrogenation on VOx over nanostructured membranes This document was produced under contract number DE-AC05-06OR23100 between the U.S. Department of Energy and Oak Ridge Associated Universities. FOREWORD The 2007 Catalysis and Chemical Transformations Program Meeting is sponsored by the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences (OBES), U.S. Department of Energy. It is being held on May 23-26, 2007, at the Wintergreen Resort, Wintergreen, VA. The main purpose of the meeting is to discuss the advances made by the program PIs over the past two years, to discuss the future directions for the program and catalysis, and to foster exchange of ideas and cooperation among participants. This year’s meeting will encompass projects in heterogeneous catalysis, surface science and electrochemistry, and some of the projects recently started in nanoscience, biocatalysis, hydrogen fuel initiative, and chemical imaging. Catalysis activities within OBES emphasize fundamental research aimed at understanding and controlling the chemical reactivity of fluid and condensed matter. -

Millerite and Other Nickel Sulfides from the Siderite Deposit „Steirischer Erzberg“, Styria, Austria
MITT. ÖSTERR. MINER. GES. 164 (2018) MILLERITE AND OTHER NICKEL SULFIDES FROM THE SIDERITE DEPOSIT „STEIRISCHER ERZBERG“, STYRIA, AUSTRIA Eugen Libowitzky*1, Anton Beran1 & Richard Göd2 1Institut für Mineralogie und Kristallographie 2 Department of Lithospheric Research Universität Wien, Althanstrasse 14, 1090 Wien / *[email protected] Abstract Millerite, NiS, has been identified for the first time in cinnabar- and pyrite-bearing siderite ore samples from „Steirischer Erzberg“, Styria, Austria. In addition, the occurrence of other nickel sulfides has been confirmed, such as siegenite-violarite solid-solutions (ss), CoNi2S4- FeNi2S4, and Gersdorffite, Ni[AsS]. Whereas milleri- te and gersdorffite are close to ideal chemistry with only minor Co and Fe contents, siegenite-violarite ss show a wide range of compositions. Almost pure siegenite with Fe below detection limit occurs in separated crystals within cinnabar or mil- lerite. In contrast, small grains (sometimes in equilibrium with millerite) within a pyrite host contain up to ~14.1 wt% Fe and thus plot in the compositional field of violarite. Zusammenfassung Millerit, NiS, wurde zum ersten mal in Zinnober- und Pyrit-führenden Sideriterz- proben vom Steirischen Erzberg, Steiermark, Österreich, nachgewiesen. Zusätzlich wurde auch das Vorkommen weitere Nickelsulfide wie Siegenit-Violarit-Mischkri- stalle (ss), CoNi2S4-FeNi2S4, und Gersdorffit, Ni[AsS], bestätigt. Während Millerit und Gersdorffit nahe der Idealchemie mit nur geringen Co- und Fe-Gehalten sind, zeigen Siegenit-Violarit ss einen weiten Zusammensetzungsbereich. Nahezu reiner Siegenit mit Fe unter der Nachweisgrenze kommt in separaten Kristallen innerhalb von Zinnober und Millerit vor. Im Gegensatz dazu enthalten kleine Einschlüsse (manchmal im Gleichgewicht mit Millerit) im Pyrit bis zu ~14.1 Gew.-% Fe und fallen damit in das Zusammensetzungsfeld von Violarit. -
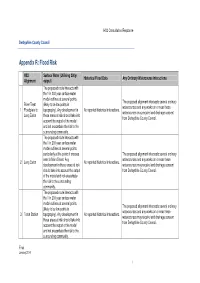
Appendix R Flood Risk Table
HS2 Consultation Response Derbyshire County Council Appendix R: Flood Risk HS2 Surface Water (Utilising 200yr Historical Flood Data Any Ordinary Watercourse interactions Alignment output) The proposed route interacts with the 1 in 200 year surface water model outlines at several points The proposed alignment intersects several ordinary River Trent (likely to be low points in watercourses and any works on or near these 1 Floodplain to topography). Any development in No reported historical interactions. watercourses may require land drainage consent Long Eaton these areas at risk should take into from Derbyshire County Council. account the output of the model and not exacerbate the risk to the surrounding community. The proposed route interacts with the 1 in 200 year surface water model outlines at several points particularly at the points it crosses The proposed alignment intersects several ordinary near to Main Street. Any watercourses and any works on or near these 2 Long Eaton No reported historical interactions. development in these areas at risk watercourses may require land drainage consent should take into account the output from Derbyshire County Council. of the model and not exacerbate the risk to the surrounding community. The proposed route interacts with the 1 in 200 year surface water model outlines at several points The proposed alignment intersects several ordinary (likely to be low points in watercourses and any works on or near these 3 Toton Station topography). Any development in No reported historical interactions. watercourses may require land drainage consent these areas at risk should take into from Derbyshire County Council. account the output of the model and not exacerbate the risk to the surrounding community.