Movement Behavior, Patch Occupancy, Sustainable Patch Networks and Conservation Planning for an Endemic Understory Bird
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter14.Pdf
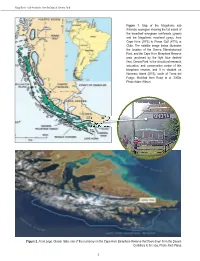
PART I • Omora Park Long-Term Ornithological Research Program THE OMORA PARK LONG-TERM ORNITHOLOGICAL RESEARCH PROGRAM: 1 STUDY SITES AND METHODS RICARDO ROZZI, JAIME E. JIMÉNEZ, FRANCISCA MASSARDO, JUAN CARLOS TORRES-MURA, AND RAJAN RIJAL In January 2000, we initiated a Long-term Ornithological Research Program at Omora Ethnobotanical Park in the world's southernmost forests: the sub-Antarctic forests of the Cape Horn Biosphere Reserve. In this chapter, we first present some key climatic, geographical, and ecological attributes of the Magellanic sub-Antarctic ecoregion compared to subpolar regions of the Northern Hemisphere. We then describe the study sites at Omora Park and other locations on Navarino Island and in the Cape Horn Biosphere Reserve. Finally, we describe the methods, including censuses, and present data for each of the bird species caught in mist nets during the first eleven years (January 2000 to December 2010) of the Omora Park Long-Term Ornithological Research Program. THE MAGELLANIC SUB-ANTARCTIC ECOREGION The contrast between the southwestern end of South America and the subpolar zone of the Northern Hemisphere allows us to more clearly distinguish and appreciate the peculiarities of an ecoregion that until recently remained invisible to the world of science and also for the political administration of Chile. So much so, that this austral region lacked a proper name, and it was generally subsumed under the generic name of Patagonia. For this reason, to distinguish it from Patagonia and from sub-Arctic regions, in the early 2000s we coined the name “Magellanic sub-Antarctic ecoregion” (Rozzi 2002). The Magellanic sub-Antarctic ecoregion extends along the southwestern margin of South America between the Gulf of Penas (47ºS) and Horn Island (56ºS) (Figure 1). -

Wild Patagonia & Central Chile
WILD PATAGONIA & CENTRAL CHILE: PUMAS, PENGUINS, CONDORS & MORE! NOVEMBER 1–18, 2019 Pumas simply rock! This year we enjoyed 9 different cats! Observing the antics of lovely Amber here and her impressive family of four cubs was certainly the highlight in Torres del Paine National Park — Photo: Andrew Whittaker LEADERS: ANDREW WHITTAKER & FERNANDO DIAZ LIST COMPILED BY: ANDREW WHITTAKER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM Sensational, phenomenal, outstanding Chile—no superlatives can ever adequately describe the amazing wildlife spectacles we enjoyed on this year’s tour to this breathtaking and friendly country! Stupendous world-class scenery abounded with a non-stop array of exciting and easy birding, fantastic endemics, and super mega Patagonian specialties. Also, as I promised from day one, everyone fell in love with Chile’s incredible array of large and colorful tapaculos; we enjoyed stellar views of all of the country’s 8 known species. Always enigmatic and confiding, the cute Chucao Tapaculo is in the Top 5 — Photo: Andrew Whittaker However, the icing on the cake of our tour was not birds but our simply amazing Puma encounters. Yet again we had another series of truly fabulous moments, even beating our previous record of 8 Pumas on the last day when I encountered a further 2 young Pumas on our way out of the park, making it an incredible 9 different Pumas! Our Puma sightings take some beating, as they have stood for the last three years at 6, 7, and 8. For sure none of us will ever forget the magical 45 minutes spent observing Amber meeting up with her four 1- year-old cubs as they joyfully greeted her return. -

Appendix 1: Maps and Plans Appendix184 Map 1: Conservation Categories for the Nominated Property
Appendix 1: Maps and Plans Appendix184 Map 1: Conservation Categories for the Nominated Property. Los Alerces National Park, Argentina 185 Map 2: Andean-North Patagonian Biosphere Reserve: Context for the Nominated Proprty. Los Alerces National Park, Argentina 186 Map 3: Vegetation of the Valdivian Ecoregion 187 Map 4: Vegetation Communities in Los Alerces National Park 188 Map 5: Strict Nature and Wildlife Reserve 189 Map 6: Usage Zoning, Los Alerces National Park 190 Map 7: Human Settlements and Infrastructure 191 Appendix 2: Species Lists Ap9n192 Appendix 2.1 List of Plant Species Recorded at PNLA 193 Appendix 2.2: List of Animal Species: Mammals 212 Appendix 2.3: List of Animal Species: Birds 214 Appendix 2.4: List of Animal Species: Reptiles 219 Appendix 2.5: List of Animal Species: Amphibians 220 Appendix 2.6: List of Animal Species: Fish 221 Appendix 2.7: List of Animal Species and Threat Status 222 Appendix 3: Law No. 19,292 Append228 Appendix 4: PNLA Management Plan Approval and Contents Appendi242 Appendix 5: Participative Process for Writing the Nomination Form Appendi252 Synthesis 252 Management Plan UpdateWorkshop 253 Annex A: Interview Guide 256 Annex B: Meetings and Interviews Held 257 Annex C: Self-Administered Survey 261 Annex D: ExternalWorkshop Participants 262 Annex E: Promotional Leaflet 264 Annex F: Interview Results Summary 267 Annex G: Survey Results Summary 272 Annex H: Esquel Declaration of Interest 274 Annex I: Trevelin Declaration of Interest 276 Annex J: Chubut Tourism Secretariat Declaration of Interest 278 -

Wildlife Travel Chile 2018
Chile, species list and trip report, 18 November to 5 December 2018 WILDLIFE TRAVEL v Chile 2018 Chile, species list and trip report, 18 November to 5 December 2018 # DATE LOCATIONS AND NOTES 1 18 November Departure from the UK. 2 19 November Arrival in Santiago and visit to El Yeso Valley. 3 20 November Departure for Robinson Crusoe (Más a Tierra). Explore San Juan Bautista. 4 21 November Juan Fernández National Park - Plazoleta del Yunque. 5 22 November Boat trip to Morro Juanango. Santuario de la Naturaleza Farolela Blanca. 6 23 November San Juan Bautista. Boat to Bahía del Padre. Return to Santiago. 7 24 November Departure for Chiloé. Dalcahue. Parque Tepuhueico. 8 25 November Parque Tepuhueico. 9 26 November Parque Tepuhueico. 10 27 November Dalcahue. Quinchao Island - Achao, Quinchao. 11 28 November Puñihuil - boat trip to Isla Metalqui. Caulin Bay. Ancud. 12 29 November Ferry across Canal de Chacao. Return to Santiago. Farellones. 13 30 November Departure for Easter Island (Rapa Nui). Ahu Tahai. Puna Pau. Ahu Akivi. 14 1 December Anakena. Te Pito Kura. Anu Tongariki. Rano Raraku. Boat trip to Motu Nui. 15 2 December Hanga Roa. Ranu Kau and Orongo. Boat trip to Motu Nui. 16 3 December Hanga Roa. Return to Santiago. 17 4 December Cerro San Cristóbal and Cerro Santa Lucía. Return to UK. Chile, species list and trip report, 18 November to 5 December 2018 LIST OF TRAVELLERS Leader Laurie Jackson West Sussex Guides Claudio Vidal Far South Expeditions Josie Nahoe Haumaka Tours Front - view of the Andes from Quinchao. Chile, species list and trip report, 18 November to 5 December 2018 Days One and Two: 18 - 19 November. -

CARMONA Et Al. (2010) Revista Chilena De Historia Natural 83: 113-142
© Sociedad de Biología de Chile SUPPLEMENTARY MATERIAL CARMONA et al. (2010) Revista Chilena de Historia Natural 83: 113-142. Senda Darwin Biological Station: Long-term ecological research at the interface between science and society Estación Biológica Senda Darwin: Investigación ecológica de largo plazo en la interfase ciencia-sociedad MARTÍN R. CARMONA 1, 2, 5 , J. C. ARAVENA 6, MARCELA A. BUSTAMANTE-SANCHEZ 1, 2 , JUAN L. CELIS-DIEZ 1, 2 , ANDRÉS CHARRIER 2, IVÁN A. DÍAZ 8, JAVIERA DÍAZ-FORESTIER 1, MARÍA F. DÍAZ 1, 10 , AURORA GAXIOLA 1, 2, 5 , ALVARO G. GUTIÉRREZ 7, CLAUDIA HERNANDEZ-PELLICER 1, 3 , SILVINA IPPI 1, 4 , ROCÍO JAÑA-PRADO 1, 2, 9 , PAOLA JARA-ARANCIO 1, 4 , JAIME JIMENEZ 13 , DANIELA MANUSCHEVICH 1, 2 , PABLO NECOCHEA 11 , MARIELA NUÑEZ-AVILA 1, 2, 8 , CLAUDIA PAPIC 11 , CECILIA PÉREZ 2, FERNANDA PÉREZ 1, 2, 5 , SHARON REID 1, 2 , LEONORA ROJAS 1, BEATRIZ SALGADO 1, 2 , CECILIA SMITH- RAMÍREZ 1, 2 , ANDREA TRONCOSO 12 , RODRIGO A. VÁSQUEZ 1, 4 , MARY F. WILLSON 1, RICARDO ROZZI 1 & JUAN J. ARMESTO 1, 2, 5, * 1 Instituto de Ecología y Biodiversidad (IEB), Facultad de Ciencias, Universidad de Chile, Las Palmeras 3425, Ñuñoa, Casilla 653, Santiago, Chile 2 Centro de Estudios Avanzados en Ecología y Biodiversidad (CASEB), Departamento de Ecología Pontificia Universidad Católica de Chile, Alameda 340, Casilla 114-D, Santiago, Chile, 833-1150 3 Centro de Estudios Avanzados en Zonas Áridas (CEAZA), Casilla 599 – Raúl Bitrán s/n, Colina El Pino, La Serena, Chile 4 Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Las Palmeras 3425, Ñuñoa, Casilla 653, Santiago, Chile 5 Laboratorio Internacional de Cambio Global (LINCGlobal), UC-CSIC, Departamento de Ecología Pontificia Universidad Católica de Chile, Alameda 340, Casilla 114-D, Santiago, Chile, 833-1150 6 Centro de Estudios del Quaternario (CEQUA), Avenida Bulnes 01890, Casilla 737, Punta Arenas, Chile 7 Department of Ecological Modelling, Helmholtz Centre for Environmental Research (UFZ), Permoserstr. -

Chile: North to Patagonia, Nov 2014
Chile: North to Patagonia, Nov 2014 Leader: Fernando Díaz Participants: Cathy Pasterczyk, Marie Carr, Neil Davis, Alain Pataud, Derek Antropik and William Porteous. Manu Expeditions Birding and Wildlife Tours www.Birding-In-Peru.com OVERVIEW…… Chile offers one the most diverse birding experiences in South America. A solid network of domestic flights and an excellent road system make it easy to get around, making it possible to visit a great variety of habitats within just a few days. Mountain passes at 4,500 meters above sea level, arid deserts, scrub, dense forests, steppes and pelagic boat rides on the Pacific Ocean are within easy reach on a journey up and down this long, thin country. The country’s geographic diversity translates into various climates, from arid in the north to Mediterranean in the center and occasionally windy and rainy farther south in Patagonia. This variety is also reflected in the diverse and tasty cuisines that can be enjoyed throughout the journey. Of course, Chile is also world famous for its exquisite wines!. The trip was divided into the three main regions of Chile: North, Central and South/Patagonia. The main goals were to visit as many habitats and to see as many bird species as possible. We included side trips to look for major target species, along with two pelagic boat rides into the rich waters of the Humboldt Current, one from Arica and the other from Valparaiso. Our group of five started in the North, and it was the first time of birding in Chile for most participants. Bill, who had been to Chile’s North on a prior visit, joined us for the Central and Patagonia sections. -

Defining Corridor Functions for Endemic Birds in Fragmented South-Temperate Rainforest
Defining Corridor Functions for Endemic Birds in Fragmented South-Temperate Rainforest KATHRYN E. SIEVING*, MARY F. WILLSON†, AND TONI L. DE SANTO‡ *Department of Wildlife Ecology and Conservation, University of Florida, 303 Newins-Ziegler Hall, Gainesville, FL 32611–0430, U.S.A., email [email protected] †The Nature Conservancy, 8 Michigan Avenue, Suite 2301, Chicago, IL 60603, U.S.A. ‡Forestry Sciences Laboratory, 2770 Sherwood Lane, Suite 2A, Juneau, AK 99801, U.S.A. Abstract: For five species of endemic understory birds ( families Rhinocryptidae, Furnariidae) inhabiting fragmented temperate rainforest in southcentral Chile, we distinguished between vegetated corridors func- tioning as living space and those potentially suitable for short-distance movements only. In the first phase of the study, we surveyed 24 forested corridors Յ50 m wide using passive and song-playback censuses. Corridor width determined species presence or absence, whereas the number of individuals increased with width and understory vegetation density. Birds were infrequently encountered in corridors Յ10 m wide but were al- ways present in corridors 25–50 m wide. Birds present in intermediate-width (11–24 m) corridors were de- tected significantly less often during passive than playback census, suggesting that these birds exhibited con- spicuous territorial display less frequently than those present in wider corridors, where passive and playback census yielded similar detection rates. Corridors approximately 10–25 m wide, therefore, may be transitional between corridors too narrow for most regular uses and those sufficiently wide for birds to establish territo- ries. Also, bird abundance decreased as the ratio of corridor length to width (L/ W) increased. -

Durham E-Theses
Durham E-Theses the spatial ecology o the Guina (Oncifelis guigna) in Southern Chile Freer, Rachel A. How to cite: Freer, Rachel A. (2004) the spatial ecology o the Guina (Oncifelis guigna) in Southern Chile, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/3050/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 Tllne §pathnll lEcollogy of tllne Grudiillla ( 0TJ1Jcifeli§ guigll1la) illll Southern ChHe by A . copyrigllnt of tll:nis til..aJtesns • rests WBtll:n t~e Bllll!Unor. No (]!lll!otation fr~m nt sholll!Jd ll>e JPlLllll>Bis.hedl Wlth~lll!t !Inns prior wrnUellll COIIllsent Rachel A. Freer Bll!Rdl mformatiollll dlerivecll from nt sll:nolll!Bdlll>e acllrnowBedlged. Department of Biological Sciences, University of Durham, UK 2004 This thesis is submitted in candidature for the degree of Doctor of Philosophy A copyrngllnt of tllnns tihlesis rests with Une aunthor. -

Gear for a Big Year
APPENDIX 1 GEAR FOR A BIG YEAR 40-liter REI Vagabond Tour 40 Two passports Travel Pack Wallet Tumi luggage tag Two notebooks Leica 10x42 Ultravid HD-Plus Two Sharpie pens binoculars Oakley sunglasses Leica 65 mm Televid spotting scope with tripod Fossil watch Leica V-Lux camera Asics GEL-Enduro 7 trail running shoes GoPro Hero3 video camera with selfie stick Four Mountain Hardwear Wicked Lite short-sleeved T-shirts 11” MacBook Air laptop Columbia Sportswear rain shell iPhone 6 (and iPhone 4) with an international phone plan Marmot down jacket iPod nano and headphones Two pairs of ExOfficio field pants SureFire Fury LED flashlight Three pairs of ExOfficio Give- with rechargeable batteries N-Go boxer underwear Green laser pointer Two long-sleeved ExOfficio BugsAway insect-repelling Yalumi LED headlamp shirts with sun protection Sea to Summit silk sleeping bag Two pairs of SmartWool socks liner Two pairs of cotton Balega socks Set of adapter plugs for the world Birding Without Borders_F.indd 264 7/14/17 10:49 AM Gear for a Big Year • 265 Wildy Adventure anti-leech Antimalarial pills socks First-aid kit Two bandanas Assorted toiletries (comb, Plain black baseball cap lip balm, eye drops, toenail clippers, tweezers, toothbrush, REI Campware spoon toothpaste, floss, aspirin, Israeli water-purification tablets Imodium, sunscreen) Birding Without Borders_F.indd 265 7/14/17 10:49 AM APPENDIX 2 BIG YEAR SNAPSHOT New Unique per per % % Country Days Total New Unique Day Day New Unique Antarctica / Falklands 8 54 54 30 7 4 100% 56% Argentina 12 435 -

THE HEART of CHILE January 25-February 8, 2020
® field guides BIRDING TOURS WORLDWIDE [email protected] • 800•728•4953 ITINERARY THE HEART OF CHILE January 25-February 8, 2020 The Inca Tern is one of the most unusual of the tern family. These beautiful birds are found along the Pacific coast of Chile and Peru, and we’ll see them when we visit the coastal areas. Photograph by participant Jane Barnette. We include here information for those interested in the 2020 Field Guides Heart of Chile tour: ¾ a general introduction to the tour ¾ a description of the birding areas to be visited on the tour ¾ an abbreviated daily itinerary with some indication of the nature of each day’s birding outings Those who register for the tour will be sent this additional material: ¾ an annotated list of the birds recorded on a previous year’s Field Guides trip to the area, with comments by guide(s) on notable species or sightings ¾ a detailed information bulletin with important logistical information and answers to questions regarding accommodations, air arrangements, clothing, currency, customs and immigration, documents, health precautions, and personal items ¾ a reference list ¾ a Field Guides checklist for preparing for and keeping track of the birds we see on the tour ¾ after the conclusion of the tour, a list of birds seen on the tour Chile is a country larger than it appears on a map; that long, thin ribbon of land along the west coast of South America is from tip to tip the distance from San Francisco to New York. THE HEART OF CHILE will focus on the central region of the country, allowing for a shorter tour with multiple night stays at most of the hotels we visit. -

Mating Success of the Endemic Des Murs' Wiretail
Mating success of the endemic Des Murs’ Wiretail (Sylviorthorhynchus desmursii, Furnariidae) in fragmented Chilean rainforests IVÁN A. DÍAZ,1,3* JUAN J. ARMESTO1 AND MARY F. WILLSON2 1Center for Advanced Studies in Ecology and Biodiversity (CASEB), Catholic University of Chile, Casilla 114-D, Santiago, Chile and Fundación ‘Senda Darwin’, Santiago, Chile (Email: diazi@ufl.edu); 25230 Te rrace Place, Juneau, Alaska and 3Department of Wildlife Ecology and Conservation, University of Florida, Gainesville, Florida, USA Abstract: We studied the effects of fragment size, vegetation structure and presence of habitat corridors on the reproductive success of the Des Murs’ Wiretail (Sylviorthorhynchus desmursii Des Murs, Furnariidae), a small (10 g) understorey bird, endemic to South American forests. In a rural landscape of Chiloé Island, southern Chile (42°S; 70°W), we determined the mating and nesting success of wiretails in 28 territories distributed in seven small (1– 20 ha) and two large (>300 ha) forest fragments during the 1997–1998 breeding season. Wiretails inhabited dense bamboo thickets in the understorey of forest patches, dense shrublands covering old fields, and dense early successional forest vegetation. Wiretails avoided open pastures. Reproductive success depended solely on the probability of finding mates, and the main factor affecting mating success was the presence of corridors. Mated individuals occupied 72% of the territories in forest patches <20 ha connected by corridors, 73% of the territories in large (>300 ha) fragments, but only 20% of territories in isolated fragments surrounded by pastures. Because of the rapid expansion of pastures in southern Chile, the conservation of wiretails and other understorey birds will depend on the maintenance of travel corridors with dense understorey vegetation between forest fragments. -

Maintaining Ecosystem Resilience: Functional Responses of Tree Cavity Nesters to Logging in Temperate Forests of the Americas
Utah State University DigitalCommons@USU Aspen Bibliography Aspen Research 2017 Maintaining ecosystem resilience: functional responses of tree cavity nesters to logging in temperate forests of the Americas Jose Tomas Ibarra Michaela Martin Kristina L. Cockle Kathy Martin Follow this and additional works at: https://digitalcommons.usu.edu/aspen_bib Part of the Agriculture Commons, Ecology and Evolutionary Biology Commons, Forest Sciences Commons, Genetics and Genomics Commons, and the Plant Sciences Commons Recommended Citation Ibarra, J. T., M. Martin, K. L. Cockle, and K. Martin. 2017. Maintaining ecosystem resilience: functional responses of tree cavity nesters to logging in temperate forests of the Americas. Scientific Reports 7:4467. This Report is brought to you for free and open access by the Aspen Research at DigitalCommons@USU. It has been accepted for inclusion in Aspen Bibliography by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. www.nature.com/scientificreports OPEN Maintaining ecosystem resilience: functional responses of tree cavity nesters to logging in temperate Received: 16 November 2016 Accepted: 19 May 2017 forests of the Americas Published: xx xx xxxx José Tomás Ibarra1,2,3, Michaela Martin1, Kristina L. Cockle1,4 & Kathy Martin1,5 Logging often reduces taxonomic diversity in forest communities, but little is known about how this biodiversity loss affects the resilience of ecosystem functions. We examined how partial logging and clearcutting of temperate forests influenced functional diversity of birds that nest in tree cavities. We used point-counts in a before-after-control-impact design to examine the effects of logging on the value, range, and density of functional traits in bird communities in Canada (21 species) and Chile (16 species).