Habitat Barriers to Movement of Understory Birds in Fragmented
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter14.Pdf
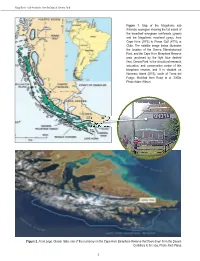
PART I • Omora Park Long-Term Ornithological Research Program THE OMORA PARK LONG-TERM ORNITHOLOGICAL RESEARCH PROGRAM: 1 STUDY SITES AND METHODS RICARDO ROZZI, JAIME E. JIMÉNEZ, FRANCISCA MASSARDO, JUAN CARLOS TORRES-MURA, AND RAJAN RIJAL In January 2000, we initiated a Long-term Ornithological Research Program at Omora Ethnobotanical Park in the world's southernmost forests: the sub-Antarctic forests of the Cape Horn Biosphere Reserve. In this chapter, we first present some key climatic, geographical, and ecological attributes of the Magellanic sub-Antarctic ecoregion compared to subpolar regions of the Northern Hemisphere. We then describe the study sites at Omora Park and other locations on Navarino Island and in the Cape Horn Biosphere Reserve. Finally, we describe the methods, including censuses, and present data for each of the bird species caught in mist nets during the first eleven years (January 2000 to December 2010) of the Omora Park Long-Term Ornithological Research Program. THE MAGELLANIC SUB-ANTARCTIC ECOREGION The contrast between the southwestern end of South America and the subpolar zone of the Northern Hemisphere allows us to more clearly distinguish and appreciate the peculiarities of an ecoregion that until recently remained invisible to the world of science and also for the political administration of Chile. So much so, that this austral region lacked a proper name, and it was generally subsumed under the generic name of Patagonia. For this reason, to distinguish it from Patagonia and from sub-Arctic regions, in the early 2000s we coined the name “Magellanic sub-Antarctic ecoregion” (Rozzi 2002). The Magellanic sub-Antarctic ecoregion extends along the southwestern margin of South America between the Gulf of Penas (47ºS) and Horn Island (56ºS) (Figure 1). -

SPLITS, LUMPS and SHUFFLES Splits, Lumps and Shuffles Alexander C
>> SPLITS, LUMPS AND SHUFFLES Splits, lumps and shuffles Alexander C. Lees This series focuses on recent taxonomic proposals—be they entirely new species, splits, lumps or reorganisations—that are likely to be of greatest interest to birders. This latest instalment includes a new Scytalopus tapaculo and a new subspecies of Three-striped Warbler, reviews of species limits in Grey-necked Wood Rails and Pearly Parakeets and comprehensive molecular studies of Buff-throated Woodcreepers, Sierra Finches, Red-crowned Ant Tanagers and Siskins. Get your lists out! Splits proposed for Grey- Pearly Parakeet is two species necked Wood Rails The three subspecies of Pearly Parakeet Pyrrhura lepida form a species complex with Crimson- The Grey-necked Wood Rail Aramides cajaneus bellied Parakeet P. perlata and replace each other is both the most widespread (occurring from geographically across a broad swathe of southern Mexico to Argentina) and the only polytypic Amazonia east of the Madeira river all the way member of its genus. Although all populations to the Atlantic Ocean. Understanding the nature are ‘diagnosable’ in having an entirely grey neck of this taxonomic variation is an important task, and contrasting chestnut chest, there is much as collectively their range sits astride much of variation in the colours of the nape, lower chest the Amazonian ‘Arc of Deforestation’ and the and mantle, differences amongst which have led to broadly-defined Brazilian endemic Pearly Parakeet the recognition of nine subspecies. Marcondes and is already considered to be globally Vulnerable. Silveira (2015) recently explored the taxonomy of Somenzari and Silveira (2015) recently investigated Grey-necked Wood Rails based on morphological the taxonomy of the three lepida subspecies (the and vocal characteristics using a sample of 800 nominate P. -

Wild Patagonia & Central Chile
WILD PATAGONIA & CENTRAL CHILE: PUMAS, PENGUINS, CONDORS & MORE! NOVEMBER 1–18, 2019 Pumas simply rock! This year we enjoyed 9 different cats! Observing the antics of lovely Amber here and her impressive family of four cubs was certainly the highlight in Torres del Paine National Park — Photo: Andrew Whittaker LEADERS: ANDREW WHITTAKER & FERNANDO DIAZ LIST COMPILED BY: ANDREW WHITTAKER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM Sensational, phenomenal, outstanding Chile—no superlatives can ever adequately describe the amazing wildlife spectacles we enjoyed on this year’s tour to this breathtaking and friendly country! Stupendous world-class scenery abounded with a non-stop array of exciting and easy birding, fantastic endemics, and super mega Patagonian specialties. Also, as I promised from day one, everyone fell in love with Chile’s incredible array of large and colorful tapaculos; we enjoyed stellar views of all of the country’s 8 known species. Always enigmatic and confiding, the cute Chucao Tapaculo is in the Top 5 — Photo: Andrew Whittaker However, the icing on the cake of our tour was not birds but our simply amazing Puma encounters. Yet again we had another series of truly fabulous moments, even beating our previous record of 8 Pumas on the last day when I encountered a further 2 young Pumas on our way out of the park, making it an incredible 9 different Pumas! Our Puma sightings take some beating, as they have stood for the last three years at 6, 7, and 8. For sure none of us will ever forget the magical 45 minutes spent observing Amber meeting up with her four 1- year-old cubs as they joyfully greeted her return. -

Systematic Relationships and Biogeography of the Tracheophone Suboscines (Aves: Passeriformes)
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 23 (2002) 499–512 www.academicpress.com Systematic relationships and biogeography of the tracheophone suboscines (Aves: Passeriformes) Martin Irestedt,a,b,* Jon Fjeldsaa,c Ulf S. Johansson,a,b and Per G.P. Ericsona a Department of Vertebrate Zoology and Molecular Systematics Laboratory, Swedish Museum of Natural History, P.O. Box 50007, SE-104 05 Stockholm, Sweden b Department of Zoology, University of Stockholm, SE-106 91 Stockholm, Sweden c Zoological Museum, University of Copenhagen, Copenhagen, Denmark Received 29 August 2001; received in revised form 17 January 2002 Abstract Based on their highly specialized ‘‘tracheophone’’ syrinx, the avian families Furnariidae (ovenbirds), Dendrocolaptidae (woodcreepers), Formicariidae (ground antbirds), Thamnophilidae (typical antbirds), Rhinocryptidae (tapaculos), and Conop- ophagidae (gnateaters) have long been recognized to constitute a monophyletic group of suboscine passerines. However, the monophyly of these families have been contested and their interrelationships are poorly understood, and this constrains the pos- sibilities for interpreting adaptive tendencies in this very diverse group. In this study we present a higher-level phylogeny and classification for the tracheophone birds based on phylogenetic analyses of sequence data obtained from 32 ingroup taxa. Both mitochondrial (cytochrome b) and nuclear genes (c-myc, RAG-1, and myoglobin) have been sequenced, and more than 3000 bp were subjected to parsimony and maximum-likelihood analyses. The phylogenetic signals in the mitochondrial and nuclear genes were compared and found to be very similar. The results from the analysis of the combined dataset (all genes, but with transitions at third codon positions in the cytochrome b excluded) partly corroborate previous phylogenetic hypotheses, but several novel arrangements were also suggested. -

A New Tapaculo Related to Scytalopus Rodriguezi from Serranía De Los Yariguíes, Colombia
Thomas M. Donegan et al. 256 Bull. B.O.C. 2013 133(4) A new tapaculo related to Scytalopus rodriguezi from Serranía de los Yariguíes, Colombia by Thomas M. Donegan, Jorge E. Avendaño & Frank Lambert Received 15 February 2013 Summary.―Upper Magdalena Tapaculo Scytalopus rodriguezi was described (in 2005) as restricted to the headwaters of the Magdalena Valley in dpto. Huila, Colombia. Here we describe a new but related taxon from the Serranía de los Yariguíes, dpto. Santander, Colombia, c.580 km to the north, which difers in its darker dorsal coloration, shorter tail, smaller body, lower mass and lower pitched song with reduced frequency bandwidth in its notes. Scytalopus tapaculos are small, primarily montane suboscines that inhabit the understorey of Neotropical forests. Species limits within the genus are problematic because of the morphological homogeneity of diferent populations, which masks a rich diversity, only detected in recent decades via vocal and genetic studies. Since vocalisations are believed to be innate and distinctive among genetically divergent Scytalopus species, and vocal diferentiation tracks molecular diferentiation more so than morphology (Arctander & Fjeldså 1994), the number of recognised species of Scytalopus has increased dramatically from ten in the mid 1990s to more than 40 today (Krabbe & Schulenberg 1997; see also, e.g., Krabbe & Schulenberg 2003, Krabbe & Cadena 2010, Hosner et al. 2013). Four new Scytalopus taxa have been described from Colombia since the late 1990s: Chocó Tapaculo S. chocoensis (Krabbe & Schulenberg 1997), Upper Magdalena Tapaculo S. rodriguezi (Krabbe et al. 2005), Stiles’ Tapaculo S. stilesi (Cuervo et al. 2005) and a subspecies of Pale-bellied Tapaculo S. -

Appendix 1: Maps and Plans Appendix184 Map 1: Conservation Categories for the Nominated Property
Appendix 1: Maps and Plans Appendix184 Map 1: Conservation Categories for the Nominated Property. Los Alerces National Park, Argentina 185 Map 2: Andean-North Patagonian Biosphere Reserve: Context for the Nominated Proprty. Los Alerces National Park, Argentina 186 Map 3: Vegetation of the Valdivian Ecoregion 187 Map 4: Vegetation Communities in Los Alerces National Park 188 Map 5: Strict Nature and Wildlife Reserve 189 Map 6: Usage Zoning, Los Alerces National Park 190 Map 7: Human Settlements and Infrastructure 191 Appendix 2: Species Lists Ap9n192 Appendix 2.1 List of Plant Species Recorded at PNLA 193 Appendix 2.2: List of Animal Species: Mammals 212 Appendix 2.3: List of Animal Species: Birds 214 Appendix 2.4: List of Animal Species: Reptiles 219 Appendix 2.5: List of Animal Species: Amphibians 220 Appendix 2.6: List of Animal Species: Fish 221 Appendix 2.7: List of Animal Species and Threat Status 222 Appendix 3: Law No. 19,292 Append228 Appendix 4: PNLA Management Plan Approval and Contents Appendi242 Appendix 5: Participative Process for Writing the Nomination Form Appendi252 Synthesis 252 Management Plan UpdateWorkshop 253 Annex A: Interview Guide 256 Annex B: Meetings and Interviews Held 257 Annex C: Self-Administered Survey 261 Annex D: ExternalWorkshop Participants 262 Annex E: Promotional Leaflet 264 Annex F: Interview Results Summary 267 Annex G: Survey Results Summary 272 Annex H: Esquel Declaration of Interest 274 Annex I: Trevelin Declaration of Interest 276 Annex J: Chubut Tourism Secretariat Declaration of Interest 278 -

04 Donegan & Avendaño-C.2008
24 Ornitología Colombiana No.6 (2008):24-65 NOTES ON TAPACULOS (PASSERIFORMES: RHINOCRYPTIDAE) OF THE EASTERN ANDES OF COLOMBIA AND THE VENEZUELAN ANDES, WITH A NEW SUBSPECIES OF SCYTALOPUS GRISEICOLLIS FROM COLOMBIA Notas sobre tapaculos (Passeriformes: Rhinocryptidae) de la Cordillera Oriental de Colombia y los Andes venezolanos, con una nueva subespecie de Scytalopus griseicollis de Colombia Thomas M. Donegan ProAves Foundation, Caversham, Reading, UK. [email protected], [email protected] Jorge Enrique Avendaño-C. 1 Escuela de Biología, Universidad Industrial de Santander, Bucaramanga, Colombia [email protected] ABSTRACT We analysed biometrics, plumage and voice and inspected specimens to study the taxonomy of various high elevation tapaculos Scytalopus of the Eastern Andes of Colombia and the Mérida Andes of Venezuela. In light of a lack of any diagnostic vocal, plumage or biometric character, we propose treating S. infasciatus as a subjective junior synonym of S. griseicollis . S. fuscicauda and S. meridanus are indistinguishable by morphology, but we propose treating S. fuscicauda as a subspecies of S. meridanus in light of small observed differences in introductions to songs, which require further investigation. As the names were published contemporaneously, we propose priority for S. meridanus over S. fuscicauda . S. meridanus and S. griseicollis as redefined are each diagnosable vocally, supporting species rank for both of them. S. griseicollis gilesi subsp. nov. is described from the Yariguíes mountains. The new subspecies differs from S. griseicollis in its darker plumage, lower acoustic frequency scolds and longer tail. The recently discovered Eastern Andes population of S. spillmanni differs from Ecuadorian populations in its shorter tarsus length and slower song, meeting the requirements for some, but not all, subspecies concepts. -

Wildlife Travel Chile 2018
Chile, species list and trip report, 18 November to 5 December 2018 WILDLIFE TRAVEL v Chile 2018 Chile, species list and trip report, 18 November to 5 December 2018 # DATE LOCATIONS AND NOTES 1 18 November Departure from the UK. 2 19 November Arrival in Santiago and visit to El Yeso Valley. 3 20 November Departure for Robinson Crusoe (Más a Tierra). Explore San Juan Bautista. 4 21 November Juan Fernández National Park - Plazoleta del Yunque. 5 22 November Boat trip to Morro Juanango. Santuario de la Naturaleza Farolela Blanca. 6 23 November San Juan Bautista. Boat to Bahía del Padre. Return to Santiago. 7 24 November Departure for Chiloé. Dalcahue. Parque Tepuhueico. 8 25 November Parque Tepuhueico. 9 26 November Parque Tepuhueico. 10 27 November Dalcahue. Quinchao Island - Achao, Quinchao. 11 28 November Puñihuil - boat trip to Isla Metalqui. Caulin Bay. Ancud. 12 29 November Ferry across Canal de Chacao. Return to Santiago. Farellones. 13 30 November Departure for Easter Island (Rapa Nui). Ahu Tahai. Puna Pau. Ahu Akivi. 14 1 December Anakena. Te Pito Kura. Anu Tongariki. Rano Raraku. Boat trip to Motu Nui. 15 2 December Hanga Roa. Ranu Kau and Orongo. Boat trip to Motu Nui. 16 3 December Hanga Roa. Return to Santiago. 17 4 December Cerro San Cristóbal and Cerro Santa Lucía. Return to UK. Chile, species list and trip report, 18 November to 5 December 2018 LIST OF TRAVELLERS Leader Laurie Jackson West Sussex Guides Claudio Vidal Far South Expeditions Josie Nahoe Haumaka Tours Front - view of the Andes from Quinchao. Chile, species list and trip report, 18 November to 5 December 2018 Days One and Two: 18 - 19 November. -

Movement Behavior, Patch Occupancy, Sustainable Patch Networks and Conservation Planning for an Endemic Understory Bird
MOVEMENT BEHAVIOR, PATCH OCCUPANCY, SUSTAINABLE PATCH NETWORKS AND CONSERVATION PLANNING FOR AN ENDEMIC UNDERSTORY BIRD By TRACI DARNELL CASTELLÓN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2006 Copyright 2006 by Traci Darnell Castellón ACKNOWLEDGMENTS I sincerely thank my advisor, Kathryn Sieving, for her unwavering support and assistance, and my graduate committee, Lyn Branch, Michael Binford, Graeme Cumming, Doug Levey, and Emelio Bruna. I am also extremely grateful to my field assistants Hector Jañez, John Davis, Alvaro Wurstten, Emma Elgueta, Juan Carlos Correra, computer assistant Nia Haynes, and the many land owners in Chiloé and Osorno who graciously provided access to their farms. This work would not have been possible without their contributions. In addition, I have appreciated and benefited from the support of my friends and colleagues Daniel Smith, Mike Milleson, Matt Reetz, Tom Contreras, Nat Seavy, Marcella Machicote, Greg Jones, and Ivan Díaz. I gratefully acknowledge Mary Willson, Juan Armesto, and Cecilia Smith. Finally, and above all, I thank my parents; Carolyn Blethen and Charles Darnell, my husband Charles Castellón, and my friends, for helping me remember what is important. Partial funding was provided by the Disney Conservation Fund. In-kind support was provided by Fundación Senda Darwin, the University of Florida Map and Image Library, Geoplan Center, the Geography Department, the Land Use and Environmental Change Institute, and the Department of Wildlife Ecology and Conservation. iii TABLE OF CONTENTS page ACKNOWLEDGMENTS ................................................................................................. iii LIST OF TABLES............................................................................................................. vi LIST OF FIGURES ......................................................................................................... -

Bird) Species List
Aves (Bird) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Archosauria, Aves Order (O:) and Family (F:) English Name2 Scientific Name3 O: Tinamiformes (Tinamous) F: Tinamidae (Tinamous) Great Tinamou Tinamus major Highland Tinamou Nothocercus bonapartei O: Galliformes (Turkeys, Pheasants & Quail) F: Cracidae Black Guan Chamaepetes unicolor (Chachalacas, Guans & Curassows) Gray-headed Chachalaca Ortalis cinereiceps F: Odontophoridae (New World Quail) Black-breasted Wood-quail Odontophorus leucolaemus Buffy-crowned Wood-Partridge Dendrortyx leucophrys Marbled Wood-Quail Odontophorus gujanensis Spotted Wood-Quail Odontophorus guttatus O: Suliformes (Cormorants) F: Fregatidae (Frigatebirds) Magnificent Frigatebird Fregata magnificens O: Pelecaniformes (Pelicans, Tropicbirds & Allies) F: Ardeidae (Herons, Egrets & Bitterns) Cattle Egret Bubulcus ibis O: Charadriiformes (Sandpipers & Allies) F: Scolopacidae (Sandpipers) Spotted Sandpiper Actitis macularius O: Gruiformes (Cranes & Allies) F: Rallidae (Rails) Gray-Cowled Wood-Rail Aramides cajaneus O: Accipitriformes (Diurnal Birds of Prey) F: Cathartidae (Vultures & Condors) Black Vulture Coragyps atratus Turkey Vulture Cathartes aura F: Pandionidae (Osprey) Osprey Pandion haliaetus F: Accipitridae (Hawks, Eagles & Kites) Barred Hawk Morphnarchus princeps Broad-winged Hawk Buteo platypterus Double-toothed Kite Harpagus bidentatus Gray-headed Kite Leptodon cayanensis Northern Harrier Circus cyaneus Ornate Hawk-Eagle Spizaetus ornatus Red-tailed -

CARMONA Et Al. (2010) Revista Chilena De Historia Natural 83: 113-142
© Sociedad de Biología de Chile SUPPLEMENTARY MATERIAL CARMONA et al. (2010) Revista Chilena de Historia Natural 83: 113-142. Senda Darwin Biological Station: Long-term ecological research at the interface between science and society Estación Biológica Senda Darwin: Investigación ecológica de largo plazo en la interfase ciencia-sociedad MARTÍN R. CARMONA 1, 2, 5 , J. C. ARAVENA 6, MARCELA A. BUSTAMANTE-SANCHEZ 1, 2 , JUAN L. CELIS-DIEZ 1, 2 , ANDRÉS CHARRIER 2, IVÁN A. DÍAZ 8, JAVIERA DÍAZ-FORESTIER 1, MARÍA F. DÍAZ 1, 10 , AURORA GAXIOLA 1, 2, 5 , ALVARO G. GUTIÉRREZ 7, CLAUDIA HERNANDEZ-PELLICER 1, 3 , SILVINA IPPI 1, 4 , ROCÍO JAÑA-PRADO 1, 2, 9 , PAOLA JARA-ARANCIO 1, 4 , JAIME JIMENEZ 13 , DANIELA MANUSCHEVICH 1, 2 , PABLO NECOCHEA 11 , MARIELA NUÑEZ-AVILA 1, 2, 8 , CLAUDIA PAPIC 11 , CECILIA PÉREZ 2, FERNANDA PÉREZ 1, 2, 5 , SHARON REID 1, 2 , LEONORA ROJAS 1, BEATRIZ SALGADO 1, 2 , CECILIA SMITH- RAMÍREZ 1, 2 , ANDREA TRONCOSO 12 , RODRIGO A. VÁSQUEZ 1, 4 , MARY F. WILLSON 1, RICARDO ROZZI 1 & JUAN J. ARMESTO 1, 2, 5, * 1 Instituto de Ecología y Biodiversidad (IEB), Facultad de Ciencias, Universidad de Chile, Las Palmeras 3425, Ñuñoa, Casilla 653, Santiago, Chile 2 Centro de Estudios Avanzados en Ecología y Biodiversidad (CASEB), Departamento de Ecología Pontificia Universidad Católica de Chile, Alameda 340, Casilla 114-D, Santiago, Chile, 833-1150 3 Centro de Estudios Avanzados en Zonas Áridas (CEAZA), Casilla 599 – Raúl Bitrán s/n, Colina El Pino, La Serena, Chile 4 Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Las Palmeras 3425, Ñuñoa, Casilla 653, Santiago, Chile 5 Laboratorio Internacional de Cambio Global (LINCGlobal), UC-CSIC, Departamento de Ecología Pontificia Universidad Católica de Chile, Alameda 340, Casilla 114-D, Santiago, Chile, 833-1150 6 Centro de Estudios del Quaternario (CEQUA), Avenida Bulnes 01890, Casilla 737, Punta Arenas, Chile 7 Department of Ecological Modelling, Helmholtz Centre for Environmental Research (UFZ), Permoserstr. -

The Birds of Páramo De Frontino, Western Andes of Colombia
Ornitología Colombiana No4 (2006): 39-50 39 THE BIRDS OF PÁRAMO DE FRONTINO, WESTERN ANDES OF COLOMBIA Aves del Páramo de Frontino, Cordillera Occidental de Colombia Niels Krabbe Zoological Museum,University of Copenhagen.Universitetsparken 15, 2100 Copenhagen, Denmark. [email protected] Pablo Flórez, Gustavo Suárez, José Castaño Fundación ProAves, Cra 20 36-61,Bogotá,Colombia. pfl [email protected], [email protected], [email protected] Juan David Arango Diagonal 75 cc # 01-110 Kalamary I tercera etapa casa 105, Medellín,Colombia. [email protected] Arley Duque Parque Nacional Las Orquídeas, Urrao, Antioquia, Colombia. ABSTRACT We conducted an ornithological survey of Páramo de Frontino, the largest páramo in the Western Andes of Colombia and rarely visited by ornithologists. Here we present the fi rst records from this cordillera of Geranoaetus melanoleucus, Hapalopsittaca amazonina, Lurocalis rufi ventris, Grallaria alleni, Myornis senilis, and Notiochelidon fl avipes, as well as Uropsalis segmentata, Acestrura mulsant, and Leptopogon rufi pectus. The latter three had been previously recorded from southern Antioquia, but had remained unpublished. We also obtained signifi cant latitudinal range extensions for 23 species and altitudinal extensions of 300 m or more are given for 26 species. The avian biogeography of the cordillera is discussed and an annotated list given of the species recorded during the survey. Key words: avian biogeography, Colombia, Páramo de Frontino, range extensions RESUMEN Realizamos una exploración ornitológica en el Páramo de Frontino, el páramo más grande de los Andes Occidentales de Colombia y rara vez visitado por los ornitólogos. Presentamos aquí los primeros registros para la cordillera de Geranoaetus melanoleucus, Hapalopsittaca amazonina, Lurocalis rufi ventris, Grallaria alleni, Myornis senilis, Notiochelidon fl avipes, así como de Uropsalis segmentata, Acestrura mulsanti y Leptopogon rufi pectus.