(12) Patent Application Publication (10) Pub. No.: US 2008/0261947 A1 Seko Et Al
Total Page:16
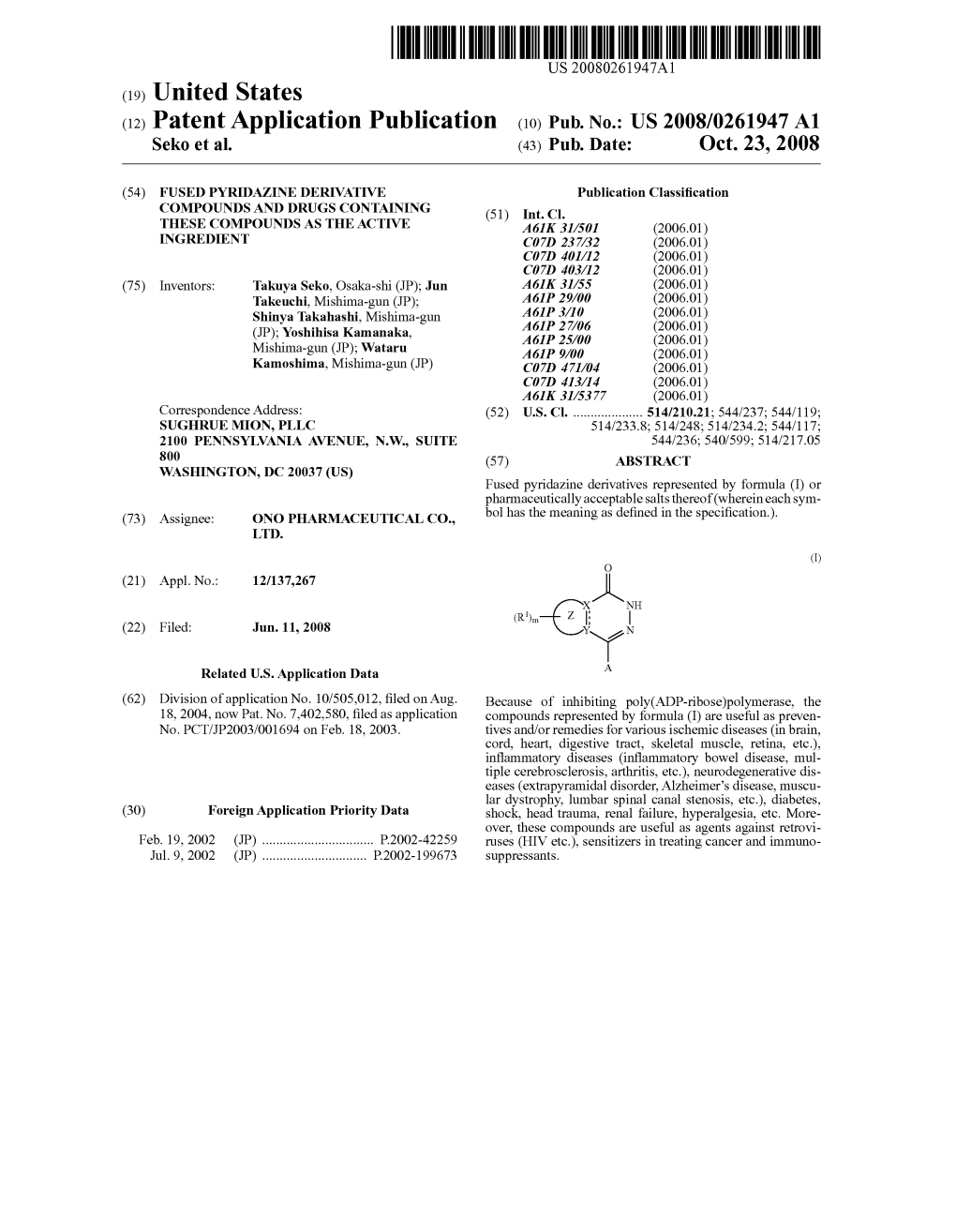
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Addition Reactions and Photoisomerization of Cis,Trans-1,5-Cyclodecadiene
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1970 Addition Reactions and Photoisomerization of Cis,trans-1,5-Cyclodecadiene. Hsin-hsiong Hsieh Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Hsieh, Hsin-hsiong, "Addition Reactions and Photoisomerization of Cis,trans-1,5-Cyclodecadiene." (1970). LSU Historical Dissertations and Theses. 1859. https://digitalcommons.lsu.edu/gradschool_disstheses/1859 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. 71-6579 HSIEH, Hsin-Hsiong, 1936- ADDITION REACTIONS AND PHOTOISOMERIZATION OF CIS,TRANS-1,5-CYCLODECADIENE. The Louisiana State University and Agricultural and Mechanical College, Ph.D., 1970 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan ADDITION REACTIONS AND PHOTOISOMERIZATION OF CIS,TRANS-1,5“CYCLODECADIENE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Hsin-Hsiong Hsieh B.S., Taiwan Provincial Chung-Hsing University, i960 M.S., Louisiana State University, -

Reaction-Of-Alkenes.Pdf
8 Reactions of Alkenes Goals for Chapter 8 1 Explain why electrophilic additions are among the most common reactions of alkenes. femoral component 2 Predict the products of the reactions of alkenes, including the orientation of the reaction (regio- polyethylene bearing chemistry) and the stereochemistry. tibial plate 3 Propose mechanisms to explain the observed products of alkene reactions. 4 Use retrosynthetic analysis to solve multistep synthesis problems with alkenes as reagents, intermediates, or products. ◀ Polyethylene provides a self-lubricating surface for movement of the metal parts of an artificial knee replacement. The highly cross-linked polyethylene used in these implants is fabricated to be exceptionally tough: It wears only about 0.1 mm per year. Polyethylene is compatible with the human body, and in most cases, it does not cause a foreign body reaction even after years of constant movement in the joint. The alkene double bond is a gateway functional group. Alkene reactions lead to many other functional groups that lay the foundation for the rest of your study of organic chemistry. You can convert alkenes to alkyl halides, epoxides, alcohols, aldehydes, ketones, carboxylic acids, and other functional groups. The reactions of alkenes arise from the reactivity of their carbon–carbon double bonds. Organic chemists enjoy the challenge of taking a simple carbon–carbon double bond and manipulating it in all possible ways to produce other compounds, often mimicking biological reactions that occur in cells. This chapter covers the most common alkene reactions, including their mechanisms, reactivity, orientation, and stereochemistry. Most reactions of alkenes involve addition of atoms or groups across the double bond, with one atom or group adding to each end. -

Photooxidation of Cyclohexene in the Presence of SO2: SOA Yield and Chemical Composition Shijie Liu1,2,3, Long Jia2, Yongfu Xu2, Narcisse T
Photooxidation of cyclohexene in the presence of SO2: SOA yield and chemical composition Shijie Liu1,2,3, Long Jia2, Yongfu Xu2, Narcisse T. Tsona1, Shuangshuang Ge2, Lin Du3,1,2 1Environment Research Institute, Shandong University, Jinan, 250100, China 5 2State Key Laboratory of Atmospheric Boundary Layer Physics and Atmospheric Chemistry, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, 100029, China 3Shenzhen Research Institute, Shandong University, Shenzhen, 518057, China Correspondence to: Lin Du ([email protected]); Yongfu Xu ([email protected]) Abstract. Secondary organic aerosol (SOA) formation from cyclohexene/NOx system with various SO2 concentrations 10 under UV light was investigated to study the effects of cyclic alkenes on the atmospheric environment in polluted urban areas. A clear decrease at first and then increase of the SOA yield was found with increasing SO2 concentrations. The lowest SOA yield was obtained when the initial SO2 concentration was in the range of 30-40 ppb, while higher SOA yield compared to that without SO2 could not be obtained until the initial SO2 concentration was higher than 85 ppb. The decreasing SOA yield might be due to the fact that the promoting effect of acid-catalyzed reactions on SOA formation was less important 15 than the inhibiting effect of decreasing OH concentration at low initial SO2 concentrations, caused by the competition reactions of OH with SO2 and cyclohexene. The competitive reaction was an important factor for SOA yield and it should not be neglected in photooxidation reactions. The composition of organic compounds in SOA was measured using several complementary techniques including Fourier transform infrared (FTIR) spectroscopy, ion chromatography (IC) and Exactive-Orbitrap mass spectrometer equipped with electro-spray interface (ESI). -

Photooxidation of Cyclohexene in the Presence of SO2: SOA Yield and Chemical Composition
Atmos. Chem. Phys., 17, 13329–13343, 2017 https://doi.org/10.5194/acp-17-13329-2017 © Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Photooxidation of cyclohexene in the presence of SO2: SOA yield and chemical composition Shijie Liu1,2,3, Long Jia2, Yongfu Xu2, Narcisse T. Tsona1, Shuangshuang Ge2, and Lin Du3,1,2 1Environment Research Institute, Shandong University, Jinan, 250100, China 2State Key Laboratory of Atmospheric Boundary Layer Physics and Atmospheric Chemistry, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, 100029, China 3Shenzhen Research Institute, Shandong University, Shenzhen, 518057, China Correspondence to: Lin Du ([email protected]) and Yongfu Xu ([email protected]) Received: 12 January 2017 – Discussion started: 15 February 2017 Revised: 28 September 2017 – Accepted: 8 October 2017 – Published: 9 November 2017 Abstract. Secondary organic aerosol (SOA) formation from 1 Introduction a cyclohexene = NOx system with various SO2 concentra- tions under UV light was investigated to study the effects Alkenes are widely emitted from biogenic and anthropogenic of cyclic alkenes on the atmospheric environment in polluted sources (Kesselmeier et al., 2002; Chin and Batterman, urban areas. A clear decrease at first and then an increase 2012), and their gas-phase oxidation reactions with OH, in the SOA yield was found with increasing SO2 concen- NO3, or O3 are among the most important processes in the trations. The lowest SOA yield was obtained when the ini- atmosphere (Atkinson, 1997; Stewart et al., 2013; Paulson tial SO2 concentration was in the range of 30–40 ppb, while et al., 1999). -

Secondary Organic Aerosol Formation from Cyclohexene Ozonolysis: Effect of OH Scavenger and the Role of Radical Chemistryseconda
Environ. Sci. Technol. 2004, 38, 3343-3350 in the cyclodecene-ozone system, in the presence of propanol Secondary Organic Aerosol scavenger, Ziemann (5) observed the formation of cyclic Formation from Cyclohexene peroxyhemiacetals, and while these products were not shown explicitly to result in an increase in aerosol yield we may Ozonolysis: Effect of OH Scavenger suppose that these large cyclic peroxyhemiacetals partition to the aerosol phase. In contrast to this, Docherty and and the Role of Radical Chemistry Ziemann (1) observed a reduction in SOA yield for the â-pinene ozonolysis when propanol scavenger was used, M. D. KEYWOOD, J. H. KROLL, compared with cyclohexane as a scavenger. V. VARUTBANGKUL, R. BAHREINI, The reaction between cyclohexene and ozone is initiated R. C. FLAGAN, AND J. H. SEINFELD* by the addition of ozone to the double bond to form a primary Departments of Environmental Science and Engineering and ozonide which stabilizes or decomposes to an excited Chemical Engineering, California Institute of Technology, bifunctional Criegee intermediate that has two isomers (5) Pasadena, California 91125 (Figure 1). How this intermediate then goes on to form SOA has been discussed extensively (5, 7-9). The predominant low molecular weight SOA products identified in the cyclo- hexene-ozone system are dicarboxylic acids and hydroxylated To isolate secondary organic aerosol (SOA) formation in dicarboxylic acids (8). Hydroxyl radicals can be produced ozone-alkene systems from the additional influence of hydroxyl from various reactions in the alkene ozonolysis mechanism. (OH) radicals formed in the gas-phase ozone-alkene The dominant pathway of OH formation is understood to be reaction, OH scavengers are employed. -

United States Patent 19 11 Patent Number: 6,156,692 Nubel Et Al
USOO6156692A United States Patent 19 11 Patent Number: 6,156,692 Nubel et al. (45) Date of Patent: *Dec. 5, 2000 54 RUTHENIUM-CONTAINING CATALYST WO 98/39346 9/1998 WIPO. COMPOSITION FOR OLEFIN METATHESIS OTHER PUBLICATIONS 75 Inventors: Philip O. Nubel; Craig Lane Hunt; C. Grunwald et al., Advance ACS Abstracts, vol. 4, No. 6, David S. Choi, all of Naperville; Tobin p. 140, Mar. 15, 1996. J. Marks, Evanston, all of Ill. Gruenwald, C., et al., “Five-Coordinate 16-Electron Car bene- and Vinylidenruthenium (II) Complexes Prepared 73 Assignee: BP Amoco Corporation, Chicago, Ill. from RuC12(C8H12)n or from the New Dihydridoruthe nium (IV) Compound RuH2C12(PiPr3)2]", Organometal * Notice: This patent is Subject to a terminal dis lics (1996), 15: 1960–1962. claimer. Demonceau, A., et al., Novel Ruthenium-Based Catalyst Systems for the Ring-Opening Metathesis Polymerization 21 Appl. No.: 08/842,251 of Low-Strain Cyclic Olefins, Macromolecules (1997), 30: 3127-3136. 22 Filed: Apr. 24, 1997 K.J. Ivin, “Summary of Catalyst Systems”, Olefin Metathe Related U.S. Application Data sis, Academic Press, New York, 1983, p. 34. 60 Provisional application No. 60/016,554, Apr. 30, 1996, and Porri, L., et al., “Ring-Opening Polymerizataion of provisional application No. 60/033,257, Dec. 6, 1996. Cycloolefins with Catalysts Derived from Ruthenium and Iridium', Die Makromolekulare Chemie, 1974, 175: (51) Int. Cl. ................................................ B01J 31/00 3097-31.15. 52 U.S. C. ... ... 502/155; 502/150, 502/152; Demonceau, A., et al., “Ruthenium-catalysed ring-opening 502/158; 502/169; 502/162 metathesis polymerization of cycloolefins initiated by dia 58 Field of Search .................................... -

Module 4 Alkenes and Alkynes Lecture 7 Alkenes 4.1 Introduction Hydrocarbons That Contain Carbon-Carbon Double Bond Are Called Alkenes (Also Called As Olefins)
NPTEL – Biotechnology – Cell Biology Module 4 Alkenes and Alkynes Lecture 7 Alkenes 4.1 Introduction Hydrocarbons that contain carbon-carbon double bond are called Alkenes (also called as Olefins). Many alkenes are found in plant and animals. It has three sp2 orbitals that lie in a plane with angles of 120°. One of the carbon–carbon bonds in a double bond is σ-bond, formed by the overlap of a sp2 orbital of one carbon with a sp2 orbital of the other carbon. The second carbon–carbon bond in the double bond is formed from side-to-side overlap of the remaining p-orbitals of the carbons. These two p-orbitals must be parallel to each other to achieve maximum orbital-orbital overlap. Therefore, all six atoms of the double- bond system are in the same plane (Figure 1). Since there is maximum side-to-side overlap, rotation about a double bond does not occur. H H C C H H Figure 1 Each C-H σ-bond is formed by overlap of a sp2 hybrid orbital of carbon atom with the 1s orbital of a hydrogen atom. The C-H bond length in ethylene is slightly shorter than the C-H bond in ethane because the sp2 orbital in ethylene has more s character that attracts the electrons even more strongly. The C=C bond in ethylene is much shorter than the C-C bond in ethane, partly because the σ-bond of ethylene is formed from sp2 orbitals and partly because both σ- and π-bonds are attracting the atoms together (Figure 2). -

The Reaction of Chlorine with Cis- and Trans- Cyclodecenes
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 The Reaction of Chlorine With Cis- And Trans- Cyclodecenes. Dewitt Boyd Stone Jr Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Stone, Dewitt Boyd Jr, "The Reaction of Chlorine With Cis- And Trans-Cyclodecenes." (1969). LSU Historical Dissertations and Theses. 1565. https://digitalcommons.lsu.edu/gradschool_disstheses/1565 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been 69" 17,129 microfilmed exactly as received STONE, Jr., DeWitt Boyd, 1939- THE REACTION OF CHLORINE WITH ci_s - AND trans ■ CYCLODECENES. Louisiana State University and Agricultural and Mechanical College, Ph.D., 1969 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. THE REACTION OF CHLORINE WITH cis- AND trans-CYCLODECENES A D issertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by DeWitt Boyd Stone, Junior B. S. in Chemical Engineering, University of Tennessee, 1962 M. S. in Organic Chemistry, University of Tennessee, 1964 January, 1969 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ACKNOWLEDGMENT The author wishes to express his deepest appreciation to Professor James G. -

The Use of Endophytic Fungi for the Conversion of Agricultural Wastes to Hydrocarbons
The use of endophytic fungi for the conversion of agricultural wastes to hydrocarbons Author: Gary A. Strobel This is a postprint of an article that originally appeared in Biofuels on July 4, 2014. Strobel, G.A. (2014) The use of endophytic fungi for the conversion of agricultural wastes to Hydrocarbons. Biofuels 5: 447-455. http://dx.doi.org/10.1080/17597269.2014.989135 Made available through Montana State University’s ScholarWorks scholarworks.montana.edu The use of endophytic fungi for the conversion of agricultural wastes to hydrocarbons Biofuels- 2014 Gary Strobel* A novel and very promising area in the search for renewable biofuels and green chemicals is the discovery of microorganisms that produce fuel-related hydrocarbons (Mycodiesel) and other useful products. This is in contrast to yeast fermentation that utilizes expensive sugars or starch to produce ethanol which is a proven and useful source of fuel, but for many reasons it is not ideal. Recently, a number of endophytic fungi have been isolated and described that make compounds such as mono- terpenoids, alkanes, cyclohexanes, cyclopentanes, and alkyl alcohols/ketones, benzenes and polyaromatic hydrocarbons. Many of these compounds are either identical to or are closely related to those specific classes of molecules that are found in diesel. Most importantly, these organisms make hydrocarbons while utilizing cellulosic and hemicellulosic polymers found in all plant-based agricultural wastes. Also discussed are the shortcomings, strategies and methods being used to further develop these technologies. *Department of Plant Sciences, Montana State University,Bozeman, Mt., 59717 and Endophytics LLC, and 920 Technology Blvd. Suite 201, Bozeman Mt., USA, 95717. -

Free Radical Additions to Cis-Cyclooctene and Cis-Cyclodecene
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1966 Free Radical Additions to Cis-Cyclooctene and Cis-Cyclodecene. Thomas Michael Couvillon Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Couvillon, Thomas Michael, "Free Radical Additions to Cis-Cyclooctene and Cis-Cyclodecene." (1966). LSU Historical Dissertations and Theses. 1115. https://digitalcommons.lsu.edu/gradschool_disstheses/1115 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dlseerletlOBhas been mlorcfllmed wcactly «• recelTwl 66—6435 C O U V I L L O N , Th o m a s Michael, 1940- FREE RADICAL ADDITIONS TO CIS- CYCLOOCTENE AND CIS-CYCLODECENE. Louisiana State University, Ph.D., 1966 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan FREE RADICAL ADDITIONS TO CIS-CYCLOOCTENE AND CIS-CYCLODECENE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Thomas Michael Couvillon B.S., Louisiana State University, Baton Rouge, 1962 January, 1966 ACKNOWLEDGMENT The author wishes to express his deepest appreciation to Professor James G. Traynham for his guidance and encour agement In this work. He would also like to thank his wife, Kay, not only for her assistance In preparing this manuscript but for her dally encouragement over the past three years which made It possible for him to c.omplete this work. -

(12) United States Patent (10) Patent No.: US 9,388,345 B2 Mcneff Et Al
USOO9388345B2 (12) United States Patent (10) Patent No.: US 9,388,345 B2 McNeff et al. (45) Date of Patent: *Jul. 12, 2016 (54) HYDROCARBON SYNTHESIS METHODS, (58) Field of Classification Search APPARATUS, AND SYSTEMS USPC ............. 585/240; 44/306, 307, 308,385,605 See application file for complete search history. (71) Applicant: SarTec Corporation, Anoka, MN (US) (56) References Cited (72) Inventors: Clayton V. McNeff, Andover, MN (US); U.S. PATENT DOCUMENTS Larry C. McNeff. Anoka, MN (US); Daniel Thomas Nowlan, Hugo, MN 2,154,835 A 4, 1939 Eisenlohr (US); Bingwen Yan, Shoreview, MN 4,138,336 A 2f1979 Mendel et al. (US); Peter G. Greuel, Anoka, MN (US) (Continued) (73) Assignee: SarTec Corporation, Anoka, MN (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this AU 2011 200090 2, 2011 patent is extended or adjusted under 35 BR 060016O2 10/2007 U.S.C. 154(b) by 0 days. (Continued) OTHER PUBLICATIONS This patent is Subject to a terminal dis claimer. Adebanjo, Adenike O. et al., “Production of Diesel-Like Fuel and Other Value-Added Chemicals from Pyrolysis of Animal Fat'. (21) Appl. No.: 13/934,713 Energy & Fuels, vol. 19, 2005, pp. 1735-1741. (Continued) (22) Filed: Jul. 3, 2013 Primary Examiner — In Suk Bullock (65) Prior Publication Data Assistant Examiner — YoungSul Jeong (74) Attorney, Agent, or Firm — Pauly, DeVries Smith & US 2014/0046104A1 Feb. 13, 2014 Deffner, L.L.C. Related U.S. Application Data (57) ABSTRACT Embodiments of the invention include apparatus and systems (60) Provisional application No. 61/667,813, filed on Jul.