Safe Upper Levels for Vitamins and Minerals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vitamin B12ointment Containing Avocado Oil in the Therapy of Plaque
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Stücker, Markus; Memmel, Ulrike; Hoffmann, Matthias; Hartung, Joachim; Altmeyer, Peter Working Paper Vitamin B12 ointment containing avocado oil in the therapy of plaque psoriasis Technical Report, No. 2001,27 Provided in Cooperation with: Collaborative Research Center 'Reduction of Complexity in Multivariate Data Structures' (SFB 475), University of Dortmund Suggested Citation: Stücker, Markus; Memmel, Ulrike; Hoffmann, Matthias; Hartung, Joachim; Altmeyer, Peter (2001) : Vitamin B12 ointment containing avocado oil in the therapy of plaque psoriasis, Technical Report, No. 2001,27, Universität Dortmund, Sonderforschungsbereich 475 - Komplexitätsreduktion in Multivariaten Datenstrukturen, Dortmund This Version is available at: http://hdl.handle.net/10419/77100 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. -

Cosmeceutical Products List
PROVEDA HERBALS Manufacturer of: - Soaps, Shampoos, Creams, Lotions, Gels, Hair Oil etc. Unit: - Plot no. 42, 43 Sector 2, Sidcul, IIE, BHEL, Ranipur, Haridwar, Uttrakhand, India Corporate office : A 42,Ashoka Crescent Road, DLF Phase 1, Gurugram, Haryana-122002 Website : www.provedaherbal.com, www.tbcbynature.in LIST OF COSMECEUTICALS PRODUCTS FACE WASH S.NO. PRODUCT NAME ACTIVE INGREDIENTS SKIN REJUVENATING & GLYCOLIC ACID, LACTIC ACID, VITAMIN-E, D-PANTHENOL (VITAMIN-B5), ALOEVERA 1 LIGHTENING FACE WASH WITH EXTRACT, GLYCERIN MILLIGLOBULES ANTI ACNE FACE WASH TEA TREE OIL, SALICYLIC ACID, ALOEVERA EXTRACT, VITAMIN-E, D-PANTHENOL, 2 WITH MILLIGLOBULES GLYCERIN & EXCIPIENTS NEEM ANTI-BACTERIAL FACE 3 NEEM OIL, ALOEVERA EXTRACT, TEA TREE OIL, AMLA EXTRACT WASH WITH MILLIGLOBULES ALOEVERA FACE WASH WITH 4 ALOEVERA EXTRACT, CUCUMBER EXTRACT, VITAMIN- E, D- PANTHENOL, ALLANTOIN CUCUMBER ALOEVERA FACE WASH WITH 5 ALOEVERA EXTRACT, WHEAT GERM EXTRACT, D-PANTHENOL, GLYCERIN MILLIGLOBULES SKIN LIGHTENING FACE WASH ARBUTIN, VITAMIN-B3 & E, GLYCOLIC ACID, LICORICE EXTRACT, AMLA EXTRACT, 6 WITH MILLIGLOBULES GLYCERIN, D-PANTHENOL 7 PAPAYA FACE WASH ALOEVERA EXTRACT, PAPAIN ENZYME, GLYCERIN, , D-PANTHENOL, VITAMIN-E 8 SALICYLIC ACID FACE WASH SALICYLIC ACID, TEA TREE OIL, ALOE VERA EXTRACT SHAMPOO ALOEVERA & VITAMIN-E ALOEVERA EXTRACT, VITAMIN-E, D-PANTHENOL, POLYQUATERNIUM-10, LEMON PEEL 1 SHAMPOO EXTRACT 2 MULTIVITAMIN SHAMPOO ALOEVERA, VITAMIN-E, D-PANTHENOL, POLYQUATERNIUM-10, LEMON PEEL EXTRACT PROTEIN & VITAMIN SHAMPOO SUN FLOWER SEED OIL, -

The Study of Influence of Nutritional Factors Supply Indicators of Blood Laboratory Animals Using Nonparametric Statistics
Advances in Zoology and Botany 1(4): 83-85, 2013 http://www.hrpub.org DOI: 10.13189/azb.2013.010403 The Study of Influence of Nutritional Factors Supply Indicators of Blood Laboratory Animals Using Nonparametric Statistics Kvan O.V.*, Lebedev S.V., Akimov S.S., Bykov A.V. Orenburg State University , Orenburg, Russia *Corresponding Author: [email protected] Copyright © 2013 Horizon Research Publishing All rights reserved. Abstract The aim of the study was to analyze using The problem of iron deficiency has a great practical non-parametric statistical method, a number of interest. According to WHO data about one third of the morphological and biochemical parameters of laboratory world population has latent iron deficiency and iron animals’ blood on a diet deficient in minerals, with deficiency anemia. additional introduction of probiotics (sporobakterin, Keen interest of researchers to this problem is connected bifidobakterin) and iron preporations with different physical not only with the prevalence of iron in nature, but with the and chemical properties. participation of its complex metabolic processes in the The studies were performed in the experimental-biological human body [9]. The fact is not of little important but the clinics (vivarium) Orenburg State University. The seventy concentration of iron is regulated with absorption female rats of Wistar strain at the age of 4-months, identical exclusively, and not with the release. An active part in the in weight, were in the period prior experience in a balanced regulation of sorption and excretion of anions, cations, water diet (feed stuff) were selected. During the experiment, the takes the intestinal microflora. -

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

Vitamin and Mineral Deficiencies Technical Situation Analysis
Vitamin and Mineral Defi ciencies Technical Situation Analysis ten year strategy for the reduction of vitamin and mineral deficiencies Vitamin and Mineral Defi ciencies Technical Situation Analysis ten year strategy for the reduction of vitamin and mineral deficiencies Produced for: Global Alliance for Improved Nutrition, Geneva By: The Academy for Educational Development (AED) 1825 Connecticut Avenue NW Washington DC 20009 Acknowledgments Th is report was written by the following research team members, who jointly developed the out- line and reviewed the drafts: Tina Sanghvi, John Fiedler, Reena Borwankar, Margaret Phillips, Robin Houston, Jay Ross and Helen Stiefel. Annette De Mattos, Stephanie Andrews, Shera Bender, Beth Daly, Kyra Lit, and Lynley Rappaport assisted the team with data and tables. Th e team takes full responsibility for the contents and conclusions of this report. Andreas Bluethner, Barbara MacDonald, Bérangère Magarinos, Amanda Marlin, Marc Van Ameringen and technical staff of GAIN/Geneva guided the team’s eff orts. Th e report could not have been completed without the contributions of the following persons, whose inputs are gratefully acknowledged: Jean Baker (AED), Karen Bell (Emory University), Bruno de Benoist (WHO), Malia Boggs (USAID), Erick Boy (MI/Ottawa), Kenneth Brown (University of California at Davis), Bruce Cogill (AED/FANTA Project), Nita Dalmiya (UNICEF/ NY), Ian Darnton-Hill (UNICEF/NY), Omar Dary (A2Z Project), Frances Davidson (USAID), Saskia de Pee (HKI), Erin Dusch (HKI), Leslie Elder (AED/FANTA Project), -

Molybdenum Studies with Sheep
MOLYBDENUM STUDIES WITH SHEEP By GIUMA M. SHERIH.A Bachelor of Science Cairo University Cairo, Egypt 1957 Submitted to the faculty of the Graduate School of the Oklahoma State Univ~rsity of Agriculture and Applied Science in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Kay, 1961 MOLYBDENUM STUDIES WITH SHEEP Thesis Approved: I Dean of the Graduate School ii OKLAHOMA STATE UNIVERSITY LIBRARY JAN 2 1962 ACKNOWLEDGEMENT The author wishes to express his deep appreciation to Dr. A. D. Tillman, Professor in the Department of Animal Husbandry, for his valu- able suggestions in planning and carrying out these studies and in writing this thesis. Grateful acknowledgement is also extended to Dr. R. J. Sirny, Associate Professor in the Department of Biochemistry for his invaluable assistance in determining the molybdenum content of the basal rations fed in these studies. 481211 iii- TABLE OF CONTENTS Page INTRODUCTION. • • • REVIEW OF LITERATURE • • • • • • • • • • 2 Molybdenum Toxicity in Ruminants. • • • • 2 Molybdenum, Copper, Sulfur, and Phosphorus Interactions. • 4 Molybdenum and Xanthine Oxidase • • • • 7 Molybdenum as a Dietary Essential for Growth. • • 13 EXPERD"iENT I • • • 1.5 Experimental Procedure. • • 15 Results and Discussion. • 1? Summary • 18 EXPERIMENT II • • • • 19 Experimental Procedure • 19 Results and Discussion. 21 Summary • • • 22 EXPER L'VIENT III • • 23 Experimental Procedure. • • • 2J Results and Discussion. • • • • • • • 24 Summary. • • 2.5 EXPERIMENT IV • • 26 Experimental Procedure. • • • • 26 Results and Discussion. • • • 0 26 Summary • • • • • 29 EXPERIMENT V. • 30 Experimental Procedure. • • • • JO Results and Discussion. • • • • J1 Summary 32 LITERATURE CITED. 33 iv LIST OF TABLES Table Page I. Perc.entage Composition of the Semi-Purified Ration •• • • • • 16 II. -

An Overview of Biosynthesis Pathways – Inspiration for Pharmaceutical and Agrochemical Discovery
An Overview of Biosynthesis Pathways – Inspiration for Pharmaceutical and Agrochemical Discovery Alan C. Spivey [email protected] 19th Oct 2019 Lessons in Synthesis - Azadirachtin • Azadirachtin is a potent insect anti-feedant from the Indian neem tree: – exact biogenesis unknown but certainly via steroid modification: O MeO C OAc O 2 H O OH O H O OH 12 O O C 11 O 14 OH oxidative 8 O H 7 cleavage highly hindered C-C bond HO OH AcO OH AcO OH for synthesis! H H of C ring H MeO2C O AcO H tirucallol azadirachtanin A azadirachtin (cf. lanosterol) (a limanoid = tetra-nor-triterpenoid) – Intense synhtetic efforts by the groups of Nicolaou, Watanabe, Ley and others since structural elucidation in 1987. –1st total synthesis achieved in 2007 by Ley following 22 yrs of effort – ~40 researchers and over 100 person-years of research! – 64-step synthesis – Veitch Angew. Chem. Int. Ed. 2007, 46, 7629 (DOI) & Veitch Angew. Chem. Int. Ed. 2007, 46, 7633 (DOI) – Review ‘The azadirachtin story’ see: Veitch Angew. Chem. Int. Ed. 2008, 47, 9402 (DOI) Format & Scope of Presentation • Metabolism & Biosynthesis – some definitions, 1° & 2° metabolites • Shikimate Metabolites – photosynthesis & glycolysis → shikimate formation → shikimate metabolites – Glyphosate – a non-selective herbicide • Alkaloids – acetylCoA & the citric acid cycle → -amino acids → alkaloids – Opioids – powerful pain killers • Fatty Acids and Polyketides –acetylCoA → malonylCoA → fatty acids, prostaglandins, polyketides, macrolide antibiotics – NSAIDs – anti-inflammatory’s • Isoprenoids/terpenes -

United States Patent (19) 11) Patent Number: 4,740,373 Kesselman Et Al
United States Patent (19) 11) Patent Number: 4,740,373 Kesselman et al. (45) Date of Patent: Apr. 26, 1988 54 STABILIZATION OF 3,932,634 1/1976 Kardys ................................ 424/237 MULTIVITAMEN/TRACE ELEMENTS 4,228,159 10/1980 MacMillan .......................... 424/145 FORMULATIONS 4,268,529 5/1981 Davis et al............................ 426/72 (75 Inventors: Morris Kesselman, Belmar, N.J.; FOREIGN PATENT DOCUMENTS Abdur R. Purkaystha, Bronx; James 0161915 11/1985 European Pat. Off............. 514/970 Cahill, Riverdale, both of N.Y. 58-198416 11/1983 Japan ................................... 514/970 (73) Assignee: USV Pharmaceutical Corporation 1080626 8/1967 United Kingdom . 21 Appl. No.: 866,842 OTHER PUBLICATIONS 22) Fied: May 27, 1986 Chem. Abst., 99:10856s (1983)-Heidt. Chem. Abst., 105:1 1967h (1986)-Vervloet et al. 51 Int. Cl.' ..................... A61K 33/34; A61K 31/07; The Effects of Ascorbic Acid and Trace Elements on A61K 31/195; A61K 31/44 Vitamin B12 Assays, J. Am. Pharm. Assoc., 43:87-90, (52) U.S. C. ...................................... 424/141; 514/52; 1954. 514/167; 514/168; 514/249; 514/251; 514/276; 514/458; 514/474; 514/499; 514/548; 514/681; Primary Examiner-Douglas W. Robinson 514/905; 514/970 (57) ABSTRACT (58) Field of Search ................. 514/167, 168, 970, 52, 514/905, 251, 276, 458,548, 681, 249, 474, 499; Disclosed are aqueous multivitamin/trace elements 424/141 formulations stabilized by a water soluble, organic acid that contains carbon-to-carbon unsaturation and water 56) References Cited soluble salts thereof selected from the group consisting U.S. PATENT DOCUMENTS of maleic acid, fumaric acid, maleamic acid and acrylic acid. -

The Effect of Vitamin Supplementation on Subclinical
molecules Review The Effect of Vitamin Supplementation on Subclinical Atherosclerosis in Patients without Manifest Cardiovascular Diseases: Never-ending Hope or Underestimated Effect? Ovidiu Mitu 1,2,* , Ioana Alexandra Cirneala 1,*, Andrada Ioana Lupsan 3, Mircea Iurciuc 4 , 5 5 2, Ivona Mitu , Daniela Cristina Dimitriu , Alexandru Dan Costache y , Antoniu Octavian Petris 1,2 and Irina Iuliana Costache 1,2 1 Department of Cardiology, Clinical Emergency Hospital “Sf. Spiridon”, 700111 Iasi, Romania 2 1st Medical Department, University of Medicine and Pharmacy “Grigore T. Popa”, 700115 Iasi, Romania 3 Department of Cardiology, University of Medicine, Pharmacy, Science and Technology, 540139 Targu Mures, Romania 4 Department of Cardiology, University of Medicine and Pharmacy “Victor Babes”, 300041 Timisoara, Romania 5 2nd Morpho-Functional Department, University of Medicine and Pharmacy “Grigore T. Popa”, 700115 Iasi, Romania * Correspondence: [email protected] (O.M.); [email protected] (I.A.C.); Tel.: +40-745-279-714 (O.M.) Medical Student, University of Medicine and Pharmacy “Grigore T. Popa”, 700115 Iasi, Romania. y Academic Editors: Raluca Maria Pop, Ada Popolo and Stefan Cristian Vesa Received: 25 March 2020; Accepted: 7 April 2020; Published: 9 April 2020 Abstract: Micronutrients, especially vitamins, play an important role in the evolution of cardiovascular diseases (CVD). It has been speculated that additional intake of vitamins may reduce the CVD burden by acting on the inflammatory and oxidative response starting from early stages of atherosclerosis, when the vascular impairment might still be reversible or, at least, slowed down. The current review assesses the role of major vitamins on subclinical atherosclerosis process and the potential clinical implications in patients without CVD. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Molybdenum in Drinking-Water
WHO/SDE/WSH/03.04/11 English only Molybdenum in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality __________________ Originally published in Guidelines for drinking-water quality, 2nd ed. Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1996. © World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications - whether for sale or for noncommercial distribution - should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization -
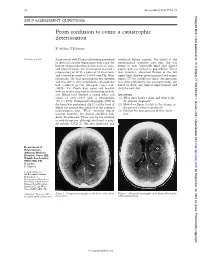
From Confusion to Coma: a Catastrophic Deterioration
52 Postgrad Med J 2001;77:52–55 Postgrad Med J: first published as 10.1136/pmj.77.903.53a on 1 January 2001. Downloaded from SELF ASSESSMENT QUESTIONS From confusion to coma: a catastrophic deterioration K Ashkan, F Johnston Answers on p 56. A previously well 45 year old woman presented ventilated before transfer. On arrival at the to the local casualty department with a one day neurosurgical intensive care unit, she was history of generalised headache, neck stiVness, found to have bilaterally fixed and dilated and blurred vision. On examination she had a pupils, with no corneal or gag reflexes. There temperature of 38°C, a pulse of 80 beats/min, was, however, abnormal flexion of the left and a blood pressure of 130/80 mm Hg. Neu- upper limb. She was given mannitol and urgent rologically, she had spontaneous eye opening repeat CT was carried out (fig 2). An operation and was able to obey commands, although she was then performed, but postoperatively she had confused speech (Glasgow coma scale failed to show any clinical improvement and (GCS), 14). Pupils were equal and reactive died the next day. with no cranial or peripheral neurological defi- cits. Blood tests showed a raised white cell Questions count of 20.9 × 109/l with a neutrophilia (1) What does figure 1 show and what is the (19.2 × 109/l). Computed tomography (CT) of diVerential diagnosis? the brain was performed (fig 1), on the basis of (2) How does figure 2 relate to the change in which the patient was referred to the regional the patient’s clinical condition? neurosurgical unit.