Documentation for All Data Sets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Phase I Trial of Tamoxifen with Ribociclib (LEE011) in Adult Patients with Advanced ER+ (HER2 Negative) Breast Cancer
The TEEL Study: A Phase I Trial of Tamoxifen with Ribociclib (LEE011) in Adult Patients with Advanced ER+ (HER2 Negative) Breast Cancer NCT02586675 Version 12.0 September 14, 2016 TEEL Protocol- Tamoxifen +Ribociclib Page 1 TITLE PAGE The TEEL Study: A Phase I trial of Tamoxifen with Ribociclib (LEE011) in adult patients with advanced ER+ (HER2 negative) breast cancer. Protocol: MCC 18332 Chesapeake IRB Pro00015228 Principal Investigator: Co-Investigators: Statistician: Experimental Therapeutics Program H. Lee Moffitt Cancer Center 12902 Magnolia Drive Tampa, FL 33612 & Comprehensive Breast Program Moffitt McKinley Outpatient Center 10920 N. McKinley Dr. Tampa, FL 33612 Study Site Contact: Protocol Version 12 Date: September 14, 2016 TEEL Protocol- Tamoxifen +Ribociclib Page 2 TITLE PAGE .............................................................................................................................................. 1 SYNOPSIS ................................................................................................................................................... 5 Patient Population ................................................................................................................................. 5 Type of Study ......................................................................................................................................... 5 Prior Therapy......................................................................................................................................... 5 -

Multi-Drug Rapid Test Panel with Adulteration (Urine)
6-mono-aceto-morphine in urine is 3-7 days. Multi-Drug Rapid Test Panel with 6-MAM 10 Adulteration (Urine) (6-MAM10) The Multi-Drug Rapid Test Panel yields a positive result when the concentration of (±) 3,4-Methylenedioxy- (±) 3,4-Methylenedioxy- benzodiazepines in urine exceeds detective level. Package Insert 500 Buprenorphine (BUP) Amphetamine(MDA500) Amphetamine Instruction Sheet for testing of any combination of the following drugs: Ethyl- β-D-Glucuronide(ETG500) Ethyl- β -D-Glucuronide 500 Buprenorphine is a potent analgesic often used in the treatment of opioid addiction. The drug is ACE/AMP/BAR/BZO/BUP/COC/THC/MTD/MET/MDMA/MOP/MQL/OPI/PCP/PPX/TCA/TML/K sold under the trade names Subutex™, Buprenex™, Temgesic™ and Suboxone™, which ET/OXY/COT/EDDP/FYL/K2/6-MAM/MDA/ETG/CLO/LSD/MPD/ZOL Ethyl- β-D-Glucuronide(ETG1,000) Ethyl- β -D-Glucuronide 1,000 contain Buprenorphine HCl alone or in combination with Naloxone HCl. Therapeutically, Including Specimen Validity Tests (S.V.T.) for: Clonazepam(CLO 400) Clonazepam 400 Buprenorphine is used as a substitution treatment for opioid addicts. Substitution treatment is a Oxidants/PCC, Specific Gravity, pH, Nitrite, Glutaraldehyde and Creatinine Clonazepam(CLO 150) Clonazepam 150 form of medical care offered to opiate addicts (primarily heroin addicts) based on a similar or A rapid test for the simultaneous, qualitative detection of multiple drugs and drug metabolites in identical substance to the drug normally used. In substitution therapy, Buprenorphine is as human urine. For healthcare professionals including professionals at point of care sites. Lysergic Acid Diethylamide (LSD) Lysergic Acid Diethylamide 20 effective as Methadone but demonstrates a lower level of physical dependence. -

Upregulation of Peroxisome Proliferator-Activated Receptor-Α And
Upregulation of peroxisome proliferator-activated receptor-α and the lipid metabolism pathway promotes carcinogenesis of ampullary cancer Chih-Yang Wang, Ying-Jui Chao, Yi-Ling Chen, Tzu-Wen Wang, Nam Nhut Phan, Hui-Ping Hsu, Yan-Shen Shan, Ming-Derg Lai 1 Supplementary Table 1. Demographics and clinical outcomes of five patients with ampullary cancer Time of Tumor Time to Age Differentia survival/ Sex Staging size Morphology Recurrence recurrence Condition (years) tion expired (cm) (months) (months) T2N0, 51 F 211 Polypoid Unknown No -- Survived 193 stage Ib T2N0, 2.41.5 58 F Mixed Good Yes 14 Expired 17 stage Ib 0.6 T3N0, 4.53.5 68 M Polypoid Good No -- Survived 162 stage IIA 1.2 T3N0, 66 M 110.8 Ulcerative Good Yes 64 Expired 227 stage IIA T3N0, 60 M 21.81 Mixed Moderate Yes 5.6 Expired 16.7 stage IIA 2 Supplementary Table 2. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of an ampullary cancer microarray using the Database for Annotation, Visualization and Integrated Discovery (DAVID). This table contains only pathways with p values that ranged 0.0001~0.05. KEGG Pathway p value Genes Pentose and 1.50E-04 UGT1A6, CRYL1, UGT1A8, AKR1B1, UGT2B11, UGT2A3, glucuronate UGT2B10, UGT2B7, XYLB interconversions Drug metabolism 1.63E-04 CYP3A4, XDH, UGT1A6, CYP3A5, CES2, CYP3A7, UGT1A8, NAT2, UGT2B11, DPYD, UGT2A3, UGT2B10, UGT2B7 Maturity-onset 2.43E-04 HNF1A, HNF4A, SLC2A2, PKLR, NEUROD1, HNF4G, diabetes of the PDX1, NR5A2, NKX2-2 young Starch and sucrose 6.03E-04 GBA3, UGT1A6, G6PC, UGT1A8, ENPP3, MGAM, SI, metabolism -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

MSM Cross Reference Antihistamine Decongestant 20100701 Final Posted
MISSISSIPPI DIVISION OF MEDICAID Antihistamine/Decongestant Product and Active Ingredient Cross-Reference List The agents listed below are the antihistamine/decongestant drug products listed in the Mississippi Medicaid Preferred Drug List (PDL). This is a cross-reference between the drug product name and its active ingredients to reference the antihistamine/decongestant portion of the PDL. For more information concerning the PDL, including non- preferred agents, the OTC formulary, and other specifics, please visit our website at www.medicaid.ms.gov. List Effective 07/16/10 Therapeutic Class Active Ingredients Preferred Non-Preferred ANTIHISTAMINES - 1ST GENERATION BROMPHENIRAMINE MALEATE BPM BROMAX BROMPHENIRAMINE MALEATE J-TAN PD BROMSPIRO LODRANE 24 LOHIST 12HR VAZOL BROMPHENIRAMINE TANNATE BROMPHENIRAMINE TANNATE J-TAN P-TEX BROMPHENIRAMINE/DIPHENHYDRAM ALA-HIST CARBINOXAMINE MALEATE CARBINOXAMINE MALEATE PALGIC CHLORPHENIRAMINE MALEATE CHLORPHENIRAMINE MALEATE CPM 12 CHLORPHENIRAMINE TANNATE ED CHLORPED ED-CHLOR-TAN MYCI CHLOR-TAN MYCI CHLORPED PEDIAPHYL TANAHIST-PD CLEMASTINE FUMARATE CLEMASTINE FUMARATE CYPROHEPTADINE HCL CYPROHEPTADINE HCL DEXCHLORPHENIRAMINE MALEATE DEXCHLORPHENIRAMINE MALEATE DIPHENHYDRAMINE HCL ALLERGY MEDICINE ALLERGY RELIEF BANOPHEN BENADRYL BENADRYL ALLERGY CHILDREN'S ALLERGY CHILDREN'S COLD & ALLERGY COMPLETE ALLERGY DIPHEDRYL DIPHENDRYL DIPHENHIST DIPHENHYDRAMINE HCL DYTUSS GENAHIST HYDRAMINE MEDI-PHEDRYL PHARBEDRYL Q-DRYL QUENALIN SILADRYL SILPHEN DIPHENHYDRAMINE TANNATE DIPHENMAX DOXYLAMINE SUCCINATE -

HMSA Drug Formulary
March 23, 2004 MEMORANDUM TO: Participating Pharmacies FROM: John T. Berthiaume, M.D. Medical Director, Pharmacy Management SUBJECT: Updated HMSA Drug Formulary Enclosed is the comprehensive updated formulary, effective April 1. This copy incorporates the changes listed in the Formulary Update sent to you in February. Please replace the current formulary sections in your pharmacy handbook with the enclosed version. If you have any questions, please call an HMSA Provider Teleservice Representative at 948- 6330 on Oahu or 1 (800) 790-4672 from the Neighbor Islands. PM04-010 HMSA DRUG FORMULARY 4/1/04 - Page 1 Code THERAPEUTIC CATEGORY LISTING 1 I. Anti-infectives A. Antibiotics 1. Penicillins - non-penicillinase resistant 2 I. Anti-infectives A. Antibiotics 2. Penicillins - penicillinase resistant 3 I. Anti-infectives A. Antibiotics 3. Cephalosporins 4 I. Anti-infectives A. Antibiotics 4. Fluoroquinolones 5 I. Anti-infectives A. Antibiotics 5. Tetracyclines 6 I. Anti-infectives A. Antibiotics 6. Macrolides 7 I. Anti-infectives A. Antibiotics 7. Vancomycin 8 I. Anti-infectives A. Antibiotics 8. Lincosamides 9 I. Anti-infectives A. Antibiotics 9. Aminoglycoside 10 I. Anti-infectives A. Antibiotics 10. Sulfonamides 11 I. Anti-Infectives A. Antibiotics 11. Vaginal preparations 12 I. Anti-infectives B. Antifungal agents 1. Oral 13 I. Anti-Infectives B. Antifungal agents 2. Vaginal preparations OTC considerations: clotrimazole (Gyne-Lotrimin 3, Mycelex-7), miconazole (Monistat 7), tioconazole (Vagistat-1), butoconazole (Femstat 3) 14 I. Anti-infectives C. Antimalarial 15 I. Anti-infectives D. Antituberculous 16 I. Anti-infectives E. Amebicides 17 I. Anti-infectives F. Antiviral agents 1. Nucleoside Reverse-transcriptase Inhibitors (NRTI) 18 I. -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -
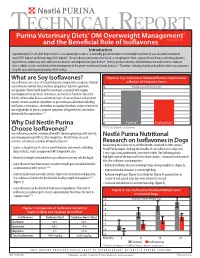
What Are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy Isoflavones Are a Class of Natural Bioactive Compounds in Soybeans
TECHNICAL REPORT Purina Veterinary Diets® OM Overweight Management® brand canine dry formula and the Beneficial Role of Isoflavones Introduction Approximately 35% of adult dogs in the U.S. are overweight or obese1. A markedly greater incidence of overweight and obesity was observed in neutered male (59% higher) and female dogs (40% higher)1. Chronic obesity can increase the risk of, or complications from, several chronic diseases including diabetes, hypertension, pulmonary and cardiovascular disease, and degenerative joint disease2. Obesity produces chronic, mild inflammation and increases oxidative stress, which, in turn, contributes to the development of the above-mentioned chronic diseases.3,4 Therefore, reducing obesity and oxidative stress can promote a long life span and improved quality of life in dogs. What are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy isoflavones are a class of natural bioactive compounds in soybeans. Natural a Marker of Oxidative Stress soy isoflavones include three chemical compounds: daidzein, genistein, 5 Plasma Isoprostanes (ng/ml) and glycitein. Many health benefits have been associated with regular consumption of soy products. In humans, soy has been found to reduce the 4 risk of cardiovascular disease and certain types of cancer (breast and prostate cancer); relieve a number of problems in post menopausal women including 3 hot flashes, osteoporosis, and decline in cognitive function; reduce cholesterol and triglycerides in plasma; improve symptoms of hypertension; and reduce 2 abdominal fat accumulation.5,6,7 1 Why Did Nestlé Purina 0 Control Isoflavones* Choose Isoflavones? *P<0.01 for Control vs. Isoflavones Soy isoflavones provide a number of benefits for managing dogs with obesity, and reducing rebound effects after weight loss. -

Applications of in Silico Methods to Analyze the Toxicity and Estrogen T Receptor-Mediated Properties of Plant-Derived Phytochemicals ∗ K
Food and Chemical Toxicology 125 (2019) 361–369 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Applications of in silico methods to analyze the toxicity and estrogen T receptor-mediated properties of plant-derived phytochemicals ∗ K. Kranthi Kumara, P. Yugandharb, B. Uma Devia, T. Siva Kumara, N. Savithrammab, P. Neerajaa, a Department of Zoology, Sri Venkateswara University, Tirupati, 517502, India b Department of Botany, Sri Venkateswara University, Tirupati, 517502, India ARTICLE INFO ABSTRACT Keywords: A myriad of phytochemicals may have potential to lead toxicity and endocrine disruption effects by interfering Phytochemicals with nuclear hormone receptors. In this examination, the toxicity and estrogen receptor−binding abilities of a QSAR modeling set of 2826 phytochemicals were evaluated. The endpoints mutagenicity, carcinogenicity (both CAESAR and ISS Toxicity models), developmental toxicity, skin sensitization and estrogen receptor relative binding affinity (ER_RBA) Nuclear hormone receptor binding were studied using the VEGA QSAR modeling package. Alongside the predictions, models were providing pos- Self−Organizing maps sible information for applicability domains and most similar compounds as similarity sets from their training Clustering and classification schemes sets. This information was subjected to perform the clustering and classification of chemicals using Self−Organizing Maps. The identified clusters and their respective indicators were considered as potential hotspot structures for the specified data set analysis. Molecular screening interpretations of models wereex- hibited accurate predictions. Moreover, the indication sets were defined significant clusters and cluster in- dicators with probable prediction labels (precision). Accordingly, developed QSAR models showed good pre- dictive abilities and robustness, which observed from applicability domains, representation spaces, clustering and classification schemes. -

Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation
www.sciencemag.org/cgi/content/full/327/5963/348/DC1 Supporting Online Material for Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation Jason Rihel,* David A. Prober, Anthony Arvanites, Kelvin Lam, Steven Zimmerman, Sumin Jang, Stephen J. Haggarty, David Kokel, Lee L. Rubin, Randall T. Peterson, Alexander F. Schier* *To whom correspondence should be addressed. E-mail: [email protected] (A.F.S.); [email protected] (J.R.) Published 15 January 2010, Science 327, 348 (2010) DOI: 10.1126/science.1183090 This PDF file includes: Materials and Methods SOM Text Figs. S1 to S18 Table S1 References Supporting Online Material Table of Contents Materials and Methods, pages 2-4 Supplemental Text 1-7, pages 5-10 Text 1. Psychotropic Drug Discovery, page 5 Text 2. Dose, pages 5-6 Text 3. Therapeutic Classes of Drugs Induce Correlated Behaviors, page 6 Text 4. Polypharmacology, pages 6-7 Text 5. Pharmacological Conservation, pages 7-9 Text 6. Non-overlapping Regulation of Rest/Wake States, page 9 Text 7. High Throughput Behavioral Screening in Practice, page 10 Supplemental Figure Legends, pages 11-14 Figure S1. Expanded hierarchical clustering analysis, pages 15-18 Figure S2. Hierarchical and k-means clustering yield similar cluster architectures, page 19 Figure S3. Expanded k-means clustergram, pages 20-23 Figure S4. Behavioral fingerprints are stable across a range of doses, page 24 Figure S5. Compounds that share biological targets have highly correlated behavioral fingerprints, page 25 Figure S6. Examples of compounds that share biological targets and/or structural similarity that give similar behavioral profiles, page 26 Figure S7. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Preventive Report Appendix
Title Authors Published Journal Volume Issue Pages DOI Final Status Exclusion Reason Nasal sumatriptan is effective in treatment of migraine attacks in children: A Ahonen K.; Hamalainen ML.; Rantala H.; 2004 Neurology 62 6 883-7 10.1212/01.wnl.0000115105.05966.a7 Deemed irrelevant in initial screening Seasonal variation in migraine. Alstadhaug KB.; Salvesen R.; Bekkelund SI. Cephalalgia : an 2005 international journal 25 10 811-6 10.1111/j.1468-2982.2005.01018.x Deemed irrelevant in initial screening Flunarizine, a calcium channel blocker: a new prophylactic drug in migraine. Amery WK. 1983 Headache 23 2 70-4 10.1111/j.1526-4610.1983.hed2302070 Deemed irrelevant in initial screening Monoamine oxidase inhibitors in the control of migraine. Anthony M.; Lance JW. Proceedings of the 1970 Australian 7 45-7 Deemed irrelevant in initial screening Prostaglandins and prostaglandin receptor antagonism in migraine. Antonova M. 2013 Danish medical 60 5 B4635 Deemed irrelevant in initial screening Divalproex extended-release in adolescent migraine prophylaxis: results of a Apostol G.; Cady RK.; Laforet GA.; Robieson randomized, double-blind, placebo-controlled study. WZ.; Olson E.; Abi-Saab WM.; Saltarelli M. 2008 Headache 48 7 1012-25 10.1111/j.1526-4610.2008.01081.x Deemed irrelevant in initial screening Divalproex sodium extended-release for the prophylaxis of migraine headache in Apostol G.; Lewis DW.; Laforet GA.; adolescents: results of a stand-alone, long-term open-label safety study. Robieson WZ.; Fugate JM.; Abi-Saab WM.; 2009 Headache 49 1 45-53 10.1111/j.1526-4610.2008.01279.x Deemed irrelevant in initial screening Safety and tolerability of divalproex sodium extended-release in the prophylaxis of Apostol G.; Pakalnis A.; Laforet GA.; migraine headaches: results of an open-label extension trial in adolescents.