Covalent Bonding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pre-Activity Quiz Answer Key (Pdf)
Name: ____________________________________________ Date: ___________________ Class: ________________________ Pre-Activity Quiz Answer Key 1. Consider a molecule of carbon monoxide (C O): A. Do you think the electrons in the triple bond pull closer to the C atom or the O atom, or are they equally shared? Use the concept of electronegativity to explain your response. Answer: They are closer to the O atom. Using the electronegativity scale from a textbook, the result is a polar covalent bond shifted towards the O atom. B. Is the bond polar or non-polar? Polar 2. In today’s engineering challenge, you will sketch out Lewis dot diagrams for various molecules and polyatomic ions. Then you will construct each molecule using a molecular model kit. The kits contain three different representations: colored balls, short sticks and long flexible springs. A. Each colored ball corresponds to a different atom. How can you determine which color to use for each atom? Use the reference sheet that comes with the kit. B. For what bond type do you think the short sticks are used? Covalent bonds C. If you were to build a triple bond, what would you use to represent a triple bond and how many would you use? Use springs, three springs 3. You will become familiar with different geometries of simple molecules. A. Name the theory used to predict molecular shapes of these molecules? VSEPR theory Molecular Models and 3-D Printing Activity—Pre-Activity Quiz Answer Key 1 Name: ____________________________________________ Date: ___________________ Class: ________________________ B. What if a molecule contains a central atom bonded to two identical outer atoms with the central atom surrounded by a lone pair of electrons? Name the geometry of this molecule. -
Review Packet- Modern Genetics Name___Page 1
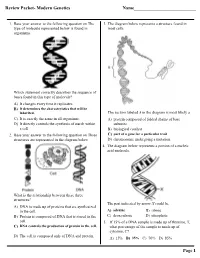
Review Packet- Modern Genetics Name___________________________ 1. Base your answer to the following question on The 3. The diagram below represents a structure found in type of molecule represented below is found in most cells. organisms. Which statement correctly describes the sequence of bases found in this type of molecule? A) It changes every time it replicates. B) It determines the characteristics that will be inherited. The section labeled A in the diagram is most likely a C) It is exactly the same in all organisms. A) protein composed of folded chains of base D) It directly controls the synthesis of starch within subunits a cell. B) biological catalyst 2. Base your answer to the following question on Three C) part of a gene for a particular trait structures are represented in the diagram below. D) chromosome undergoing a mutation 4. The diagram below represents a portion of a nucleic acid molecule. What is the relationship between these three structures? The part indicated by arrow X could be A) DNA is made up of proteins that are synthesized in the cell. A) adenine B) ribose B) Protein is composed of DNA that is stored in the C) deoxyribose D) phosphate cell. 5. If 15% of a DNA sample is made up of thymine, T, C) DNA controls the production of protein in the cell. what percentage of the sample is made up of cytosine, C? D) The cell is composed only of DNA and protein. A) 15% B) 35% C) 70% D) 85% Page 1 6. Base your answer to the following question on Which 10.The diagram below represents genetic material. -

Application of X-Ray Diffraction Methods and Molecular Mechanics Simulations to Structure Determination and Cotton Fiber Analysis
University of New Orleans ScholarWorks@UNO University of New Orleans Theses and Dissertations Dissertations and Theses 12-19-2008 Application of X-ray Diffraction Methods and Molecular Mechanics Simulations to Structure Determination and Cotton Fiber Analysis Zakhia Moore University of New Orleans Follow this and additional works at: https://scholarworks.uno.edu/td Recommended Citation Moore, Zakhia, "Application of X-ray Diffraction Methods and Molecular Mechanics Simulations to Structure Determination and Cotton Fiber Analysis" (2008). University of New Orleans Theses and Dissertations. 888. https://scholarworks.uno.edu/td/888 This Dissertation is protected by copyright and/or related rights. It has been brought to you by ScholarWorks@UNO with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in University of New Orleans Theses and Dissertations by an authorized administrator of ScholarWorks@UNO. For more information, please contact [email protected]. Application of X-ray Diffraction Methods and Molecular Mechanics Simulations to Structure Determination and Cotton Fiber Analysis A Dissertation Submitted to the Graduate Faculty of the University of New Orleans in partial fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Department of Chemistry by Zakhia Moore B.S., Xavier University, 2000 M.S., University of New Orleans, 2005 December, 2008 ACKNOWLEDGEMENTS Giving all glory to God for His many blessings, whom without I could not have completed this long journey. -

Computational Studies of Three Chemical Systems
Computational Studies of Three Chemical Systems _______________________________________ A Dissertation Presented to The Faculty of the Graduate School University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy _____________________________________________________ by Haunani Thomas Prof. Carol A. Deakyne, Dissertation Supervisor December 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled COMPUTATIONAL STUDIES OF THREE CHEMICAL SYSTEMS Presented by Haunani Thomas, a candidate for the degree of doctor of philosophy of Chemistry, and hereby certify that, in their opinion, it is worthy of acceptance. Professor Carol Deakyne (Chair) Professor John Adams (Member) Professor Michael Greenlief (Member) Professor Giovanni Vignale (Outside Member) ACKNOWLEDGEMENTS I am forever indebted to my dissertation supervisor, Professor Carol Deakyne, for her guidance and support. She has been an excellent and patient mentor through the graduate school process, always mindful of the practical necessities of my progress, introducing me to the field of Chemistry, and allowing me to advance professionally through attendance and presentations at ACS meetings. I am fully aware that, in this respect, not all graduate students are as fortunate as I have been. I am also grateful to Professor John Adams for all his support and teaching throughout my graduate career. I am appreciative of my entire committee, Professor Deakyne, Professor Adams, Professor Michael Greenlief, and Professor Giovanni Vignale, for their sound advice, patience, and flexibility. I would also like to thank my experimental collaborators and their groups, Professor Joel Liebman of the University of Maryland, Baltimore County, Professor Michael Van Stip Donk of Wichita State University, and Professor Jerry Atwood of the University of Missouri – Columbia. -

IAPS Act 15-17 Section Preview of the Teachers Edition.Pdf
Section Preview of the Teacher’s Edition for The Chemistry of Materials, Issues and Physical Science, 2nd Edition Activities 15-17 Suggested student responses and answer keys have been blocked out so that web-savvy students do not find this page and have access to answers. To experience a complete activity please request a sample found in the footer at lab-aids.com Families of Elements 15 4 s 0 2 n - o t si o es 50 te s -minu N O I T I N A ACTIVITY OVERVIEW V E S T I G Students compare physical and chemical properties of 13 elements and sort them into groups based on common properties. They then compare their classifications with groups—or families—of elements as defined by scientists and displayed in the Periodic Table of the Elements. KEY CONCEPTS AND PROCESS SKILLS (with correlation to NSE 5–8 Content Standards) 1. Elements have characteristic physical and chemical properties. Substances often are placed in categories or groups if they react in similar ways; metals are an example of such a group. (PhysSci: 1) 2. Scientists classify elements into families, based on their properties. (PhysSci: 1) 3. Students develop descriptions based on evidence. (Inquiry: 1) KEY VOCABULARY atom atomic mass element family (of elements) metal Periodic Table of the Elements B-39 Activity 15 • Families of Elements MATERIALS AND ADVANCE PREPARATION For the teacher aluminum, iron, copper, and carbon samples from Activity 14, “Physical and Chemical Properties of Materials” 1 Transparency 15.1, “Information on Element Cards” 8 sets of 4 Element Family Cards -

A New Way for Probing Bond Strength J
A New Way for Probing Bond Strength J. Klein, H. Khartabil, J.C. Boisson, J. Contreras-Garcia, Jean-Philip Piquemal, E. Henon To cite this version: J. Klein, H. Khartabil, J.C. Boisson, J. Contreras-Garcia, Jean-Philip Piquemal, et al.. A New Way for Probing Bond Strength. Journal of Physical Chemistry A, American Chemical Society, 2020, 124 (9), pp.1850-1860. 10.1021/acs.jpca.9b09845. hal-02377737 HAL Id: hal-02377737 https://hal.archives-ouvertes.fr/hal-02377737 Submitted on 27 Mar 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A New Way for Probing Bond Strength Johanna Klein,y Hassan Khartabil,y Jean-Charles Boisson,z Julia Contreras-Garc´ıa,{ Jean-Philip Piquemal,{ and Eric H´enon∗,y yInstitut de Chimie Mol´eculaire de Reims UMR CNRS 7312, Universit´ede Reims Champagne-Ardenne, Moulin de la Housse 51687 Reims Cedex 02 BP39 (France) zCReSTIC EA 3804, Universit´ede Reims Champagne-Ardenne, Moulin de la Housse 51687 Reims Cedex 02 BP39 (France) {Sorbonne Universit´es,UPMC, Laboratoire de Chimie Th´eoriqueand UMR CNRS 7616, 4 Pl Jussieu, 75252 Paris Cedex 05(France) E-mail: [email protected] Phone: +33(3)26918497 1 Abstract The covalent chemical bond is intimately linked to electron sharing between atoms. -

Searching Coordination Compounds
CAS ONLINEB Available on STN Internationalm The Scientific & Technical Information Network SEARCHING COORDINATION COMPOUNDS December 1986 Chemical Abstracts Service A Division of the American Chemical Society 2540 Olentangy River Road P.O. Box 3012 Columbus, OH 43210 Copyright O 1986 American Chemical Society Quoting or copying of material from this publication for educational purposes is encouraged. providing acknowledgment is made of the source of such material. SEARCHING COORDINATION COMPOUNDS prepared by Adrienne W. Kozlowski Professor of Chemistry Central Connecticut State University while on sabbatical leave as a Visiting Educator, Chemical Abstracts Service Table of Contents Topic PKEFACE ............................s.~........................ 1 CHAPTER 1: INTRODUCTION TO SEARCHING IN CAS ONLINE ............... 1 What is Substructure Searching? ............................... 1 The Basic Commands .............................................. 2 CHAPTEK 2: INTKOOUCTION TO COORDINATION COPPOUNDS ................ 5 Definitions and Terminology ..................................... 5 Ligand Characteristics.......................................... 6 Metal Characteristics .................................... ... 8 CHAPTEK 3: STKUCTUKING AND REGISTKATION POLICIES FOR COORDINATION COMPOUNDS .............................................11 Policies for Structuring Coordination Compounds ................. Ligands .................................................... Ligand Structures........................................... Metal-Ligand -

Molecular Model-Building by Computer
Molecular Model-building by Computer In which biochemists observe models of giant molecules as they are displayed on a screen by a computer and try to fold them into the shapes that they assume in nature by Cyrus Levinthal any problems of modern biology a large number of problems in biology computer to help gain such information are concerned with the detailed into which biologists have some insight about the structure of large biological M relation between biological but concerning which they cannot yet molecules. function and molecular structure. Some ask suitable questions in terms of molec- For the first half of this century the of the questions currently being asked ular structure. As they see such prob- metabolic and structural relations among will be completely answered only when lems more clearly, however, they in- the small molecules of the living cell one has an understanding of the struc- variably find an increasing need for were the principal concern of bio- ture of all the molecular components of structural information. In our laboratory chemists. The chemical reactions these a biological system and a knowledge of at the Massachusetts Institute of Tech- molecules undergo have been studied how they interact. There are, of course, nology we have recently started using a intensively. Such reactions are specifical- ly catalyzed by the large protein mole- cules called enzymes, many of which have now been purified and also studied. It is only within the past few years, however, that X-ray-diffraction techniques have made it possible to determine the molecular structure of such protein molecules. -

Coordinate Covalent C F B Bonding in Phenylborates and Latent Formation of Phenyl Anions from Phenylboronic Acid†
J. Phys. Chem. A 2006, 110, 1295-1304 1295 Coordinate Covalent C f B Bonding in Phenylborates and Latent Formation of Phenyl Anions from Phenylboronic Acid† Rainer Glaser* and Nathan Knotts Department of Chemistry, UniVersity of MissourisColumbia, Columbia, Missouri 65211 ReceiVed: July 4, 2005; In Final Form: August 8, 2005 The results are reported of a theoretical study of the addition of small nucleophiles Nu- (HO-,F-)to - phenylboronic acid Ph-B(OH)2 and of the stability of the resulting complexes [Ph-B(OH)2Nu] with regard - - - - - to Ph-B heterolysis [Ph-B(OH)2Nu] f Ph + B(OH)2Nu as well as Nu /Ph substitution [Ph-B(OH)2Nu] - - - + Nu f Ph + [B(OH)2Nu2] . These reactions are of fundamental importance for the Suzuki-Miyaura cross-coupling reaction and many other processes in chemistry and biology that involve phenylboronic acids. The species were characterized by potential energy surface analysis (B3LYP/6-31+G*), examined by electronic structure analysis (B3LYP/6-311++G**), and reaction energies (CCSD/6-311++G**) and solvation energies - (PCM and IPCM, B3LYP/6-311++G**) were determined. It is shown that Ph-B bonding in [Ph-B(OH)2Nu] is coordinate covalent and rather weak (<50 kcal‚mol-1). The coordinate covalent bonding is large enough to inhibit unimolecular dissociation and bimolecular nucleophile-assisted phenyl anion liberation is slowed greatly by the negative charge on the borate’s periphery. The latter is the major reason for the extraordinary differences in the kinetic stabilities of diazonium ions and borates in nucleophilic substitution reactions despite their rather similar coordinate covalent bond strengths. -

CORINA Classic Program Manual
CORINA Classic Generation of Three-Dimensional Molecular Models Version 4.4.0 Program Description Molecular Networks GmbH September 2021 www.mn-am.com Molecular Networks GmbH Altamira LLC Neumeyerstraße 28 470 W Broad St, Unit #5007 90411 Nuremberg Columbus, OH 43215 Germany USA mn-am.com This document is copyright © 1998-2021 by Molecular Networks GmbH Computerchemie and Altamira LLC. All rights reserved. Except as permitted under the terms of the Software Licensing Agreement of Molecular Networks GmbH Computerchemie, no part of this publication may be reproduced or distributed in any form or by any means or stored in a database retrieval system without the prior written permission of Molecular Networks GmbH or Altamira LLC. The software described in this document is furnished under a license and may be used and copied only in accordance with the terms of such license. (Document version: 4.4.0-2021-09-30) Content Content 1 Introducing CORINA Classic 1 1.1 Objective of CORINA Classic 1 1.2 CORINA Classic in Brief 1 2 Release Notes 3 2.1 CORINA (Full Version) 3 2.2 CORINA_F (Restricted FlexX Interface Version) 25 3 Getting Started with CORINA Classic 26 4 Using CORINA Classic 29 4.1 Synopsis 29 4.2 Options 29 5 Use Cases of CORINA Classic 60 6 Supported File Formats and Interfaces 64 6.1 V2000 Structure Data File (SD) and Reaction Data File (RD) 64 6.2 V3000 Structure Data File (SD) and Reaction Data File (RD) 67 6.3 SMILES Linear Notation 67 6.4 InChI file format 69 6.5 SYBYL File Formats 70 6.6 Brookhaven Protein Data Bank Format (PDB) -

Chapter 19 D-Block Metal Chemistry: General Considerations
Chapter 19 d-block metal chemistry: general considerations Ground state electronic configurations Reactivity, characteristic properties Electroneutrality principle Kepert Model Coordination Numbers Isomerism Electron configurations Exceptions: Cr, Cu, Nb, Mo, Au, La, Ce, and others 1 Trends in metallic radii (rmetal) across the three rows of s- and d-block metals Lanthanide contraction Cr Fe Mn 2 First Ionization energies Standard reduction potentials (298 K) 3 Reactivity of Metals Os 2O2 OsO4 Fe S FeS n V X VX (X F,n 5; X Cl,n 4; X Br,I,n 3) 2 2 n October 27, 2014 http://cen.acs.org/articles/92/i43/Iridium-Dressed-Nines.html Oxidation States Most stable states are marked in blue. An oxidation state enclosed in [ ] is rare. 4 MnCO3 NiSO4 K3Fe(CN)6 CuSO4▪5H2O CoCl2 CoCl2▪6H2O 2 2 CrK(SO ) •12H O [Co(OH 2 )6 ] 4Cl [CoCl4 ] 6H2O 4 2 2 pink blue Chromophore: the group of atoms in a molecule responsible for the absorption of electromagnetic radiation. Colors of d-block metal compounds For a single absorption in the visible region, the color you see is the complementary color of the light absorbed. •Many of the colors of low intensity are consistent with electronic d-d transitions. •In an isolated gas phase ion, such transitions would be forbidden by the Laporte selection rule, which states Δl = ± 1 where l is the orbital quantum number. - •Intense colors of species such as [MnO4] have a different origin, namely charge transfer (CT) absorptions or emissions. These are not subject to the Laporte selection rule. -

You Cannot Use a Red Pen to Take the Exam
Chemistry 320M/328M NAME (Print): _____________________________ Dr. Brent Iverson Final December 16, 2019 SIGNATURE: _____________________________ EID: _________________ Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so you can find ones you can answer and show me what you know, rather than just a few questions that may be testing the one thing you forgot. I recommend you look the exam over and answer the questions you are sure of first, then go back and try to figure out the rest. Also make sure to look at the point totals on the questions as a guide to help budget your time. You cannot use a red pen to take the exam. You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded. Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process. FINALLY, DUE TO SOME UNFORTUNATE RECENT INCIDENCTS YOU ARE NOT ALLOWED TO INTERACT WITH YOUR CELL PHONE IN ANY WAY. IF YOU TOUCH YOUR CELL PHONE DURING THE EXAM YOU WILL GET A "0" NO MATTER WHAT YOU ARE DOING WITH THE PHONE. PUT IT AWAY AND LEAVE IT THERE!!! Page Points 1 (29) 2 (23) 3 (24) 4 (24) 5 (-) 6 (-) 7 (-) 8 (28) 9 (21) 10 (23) 11 (26) 12 (27) 13 (32) 14 (32) 15 (33) 16 (16) 17 (11) 18 (10) 19 (19) 20 (10) 21 (14) Total (402) Take a deep breath and begin working.