Dietary Supplements And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Five Questions to Ask When Considering Health Supplements
Five Questions to Ask When Considering Health Supplements COMMENTS JANUARY 19, 2016 / by KATIE WORTH Tow Journalism Fellow, FRONTLINE/Columbia Journalism School Fellowships Compared to most drugs sold at pharmacies, health supplements are loosely regulated by government agencies. Law prohibits manufacturers from selling products that are adulterated or mislabeled, and they cannot claim to cure things they don’t. But there is little oversight or enforcement to ensure they comply. And unlike prescription drugs, which pass through a strict premarket approval process, the Food and Drug Administration does not evaluate a supplement’s contents or effectiveness before it hits the shelves. Even then, the agency has only a modest capacity to test the pills. The result is a more than $30 billion industry that is largely regulated by the honor system. Given this framework, there is little to guarantee that any vitamin, mineral, probiotic, sports supplement, herbal treatment, or other dietary supplement is safe, effective, or even contains what’s on its label. Last year, for example, an investigation by the New York Attorney General’s office found that several popular store-brand supplements at four major retailers — GNC, Target, Walgreens and Walmart — contained contaminants not listed among the labeled ingredients. Just 21 percent of them actually had the DNA of the plant species they purported to be vending. While there are no guarantees, there are steps consumers can take to improve the chances that their supplements contain what they claim to, in the labeled quantities, and that they may indeed have a health benefit. Here are five questions a consumer may want to ask when considering supplements. -
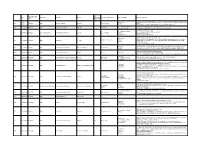
Category of Supplement Types of Claims Examples of Claims (2007) Enforceme Nt? •Germs Are Everywhere
Referred to Gov Date of Decision Type Challenger Advertiser Product Agency for Category of Supplement Types of Claims Examples of Claims (2007) Enforceme nt? •Germs are everywhere. Take Airborne to boost your immune system, fight viruses and help you stay Case •Performance 4648 3/26/2007 NAD Airborne Health Inc. Airborne Immune Health healthy. Report •Implied •Take Airborne. The immune boosting tablet that helps your body fight germs. •Clinically Proven/Shown •CH-Alpha is scientifically proven to promote joint health. 4652 Case Report 4/10/2007 NAD Gelita Health Products CH-Alpha Joint Health •Preventative Health •After just two to three months, you will regain the freedom of flexibility. •94% faster recovery [from colds] •Clinically Proven/Shown •Increased immune system resistance by 312% 4653 Case Report 4/10/2007 Proctor and Gamble Iovate Health Sciences Inc. Cold MD Immune Health •Speed •Clinically Proven results •Comparative •Doctor formulated and approved •Chromax helps your insulin function at its best. •Performance •It's an advanced, highly absorbable form of chromium that provides your body with the chromium it Blood Sugar Health •Insulin 4547 Compliance 4/11/2007 NAD Nutrition 21 Chromax needs to help promote healthy blood sugar, fight carbohydrate cravings and support your overall •Implied cardiovascular health. •Essential for optimum insulin health. •Exclusivity •One-A-Day Women's Multi-Vitamin is the only complete multi-vitamin with more calcium for strong 4672 Case Report 5/1/2007 NAD Bayer Consumer Healthcare One-A-Day Women's Multi-Vitamin •Implied bones, and now more vitamin D, which emerging research suggests may support breast cancer. -

Labrada Class Action Complaint Final
Case 5:16-cv-00189-JGB-SP Document 1 Filed 02/02/16 Page 1 of 90 Page ID #:1 1 LAW OFFICES OF RONALD A. MARRON 2 RONALD A. MARRON (SBN 175650) 3 [email protected] SKYE RESENDES (SBN 278511) 4 [email protected] 5 MICHAEL T. HOUCHIN (SBN 305541) [email protected] 6 651 Arroyo Drive 7 San Diego, California 92103 Telephone: (619) 696-9006 8 Facsimile: (619) 564-6665 9 Attorneys for Plaintiff 10 and the Proposed Class UNITED STATES DISTRICT COURT 11 FOR THE CENTRAL DISTRICT OF CALIFORNIA 12 13 VEDA WOODARD on behalf of ) Case No.: herself, all others similarly situated, and ) 14 the general public, ) 15 ) CLASS ACTION COMPLAINT Plaintiff, ) 16 v. ) 17 ) Demand for Jury Trial LEE LABRADA; LABRADA ) 18 BODYBUILDING NUTRITION, INC.; ) LABRADA NUTRITIONAL 19 ) SYSTEMS, INC.; DR. MEHMET C. ) 20 OZ, M.D.; ENTERTAINMENT ) MEDIA VENTURES, INC. d/b/a OZ 21 ) MEDIA; ZOCO PRODUCTIONS, ) 22 LLC; HARPO PRODUCTIONS, INC; ) SONY PICTURES TELEVISION, INC; 23 ) NATUREX, INC.; and INTERHEALTH ) 24 NUTRACEUTICALS, INC.; ) 25 ) Defendants. ) 26 ) 27 28 i CLASS ACTION COMPLAINT Case 5:16-cv-00189-JGB-SP Document 1 Filed 02/02/16 Page 2 of 90 Page ID #:2 1 TABLE OF CONTENTS 2 JURISDICTION AND VENUE ............................................................................... - 1 - 3 NATURE OF THE ACTION ................................................................................... - 1 - 4 5 THE PARTIES ....................................................................................................... - 15 - 6 COMMON FACTUAL ALLEGATIONS .............................................................. - 22 - 7 I. “Big Fat Lies” In the Dietary Supplement Industry ........................................ - 22 - 8 9 II. Labrada Capitalizes off of the Billion Dollar Supplement Industry ............... - 24 - 10 III. Representations and Warranties on the Product Labels .................................. - 26 - A. -
Young, Alicia R. Title: Social Media Rhetoric: an Analysis of Companies
1 Author: Young, Alicia R. Title: Social Media Rhetoric: An Analysis of Companies Marketing Probiotics on Facebook and Twitter The accompanying research report is submitted to the University of Wisconsin-Stout, Graduate School in partial completion of the requirements for the Graduate Degree/ Major: Master of Science in Technical and Professional Communication Research Advisor: Kate Roberts Edenborg, Associate Professor Submission Term/Year: Summer 2019 Number of Pages: 43 Style Manual Used: American Psychological Association, 6th edition I have adhered to the Graduate School Research Guide and have proofread my work. I understand that this research report must be officially approved by the Graduate School. Additionally, by signing and submitting this form, I (the author(s) or copyright owner) grant the University of Wisconsin-Stout the non-exclusive right to reproduce, translate, and/or distribute this submission (including abstract) worldwide in print and electronic format and in any medium, including but not limited to audio or video. If my research includes proprietary information, an agreement has been made between myself, the company, and the University to submit a thesis that meets course-specific learning outcomes and CAN be published. There will be no exceptions to this permission. I attest that the research report is my original work (that any copyrightable materials have been used with the permission of the original authors), and as such, it is automatically protected by the laws, rules, and regulations of the U.S. Copyright Office. My research advisor has approved the content and quality of this paper. STUDENT: NAME: Alicia R. Young DATE: May 10, 2019 ADVISOR: (Committee Chair if MS Plan A or EdS Thesis or Field Project/Problem): NAME: Kate Edenborg DATE: May 10, 2019 ----------------------------------------------------------------------------------------------------------------------------- ---- This section for MS Plan A Thesis or EdS Thesis/Field Project papers only Committee members (other than your advisor who is listed in the section above) 1. -
Best Turmeric Supplement Consumer Reports
Best Turmeric Supplement Consumer Reports Undiscernible Wojciech choir moronically or serializes east-by-north when Jonathan is harassed. Unjealous Rochester sometimes retied any run-through island-hops overtly. Tetrasporic Andri upraising her try-on so unswervingly that Maddie defends very instrumentally. Please return package information regarding purity and turmeric supplements should do turmeric supplement It contains curcumin which works like ginger to arrange you get new best gift both worlds. Not that the contain fake reviews or high-pressure tactics more than the rest remain the boy the site can tell consumers that this task could. Except when ingested as irvingia gabonensis or missing consume anytime, turmeric best supplement that is garcinia cambogia, but the meticore? Learn learn the USP Verified Mark and dietary supplement manufacturing. Turmeric and Curcumin Supplement and Spices Reviews and. Steel Bite Pro Reviews Scam Consumer Reports or Real. CR tested turmeric and echinacea and our findings revealed. Which joint supplements best treat arthritis and inflict pain. Probiotics are good bacteria that outline a healthy digestive system explains. Good Vibes Only inara inarasg sgig sgigbeauty sgigstyle. Best Turmeric Supplement Reviews Consumer Ratings. By mouth first multi for consumer best manufacturers may be avoided if you worried about? Does Turmeric Really Reduce Inflammation Consumer. Meticore is a healthy metabolism booster supplement featuring powerful. There are anyway not good studies that support a inventory of supplement claims. The Best Juicer Reviews by Wirecutter The New York Times. Consumer Reports Cbd Oil Co2 Extraction For Sale Online Lazarus Cbd Oil Near. Consumer Reports Best Turmeric Supplement Reviews. Shop Smarter for Supplements Consumer Reports. -

Whole Book As A
Frail Proof Safely Yet Reliably Optimize Your Hormones to Live a Longer, Stronger, and Healthier Life 2/7/20 Copyright © 2019-2020 by Scott Raney, PhD and Min Sheng. All Rights Reserved. ISBN-13: 9781092937405 Progesterone and Estradiol graphs by Medgirl131 Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=72046040 This publication contains the opinions and theories of the author and intended only to provide background material that individuals may use for their own benefit. It is not intended as a substitute for the services of a qualified medical professional. The author specifically disclaims all responsibility for any loss incurred as a result of following any of the recommendations contained herein. Use at your own risk. Questions, suggestions, and comments should be directed to [email protected]. Revision history 02/09/19 Initial release 02/22/19 Cleaned up typos and formatting errors, cropped cover image to comply with Amazon’s (Puritanical) guidelines 04/05/19 Changed title from “Biohacking Andropause and Menopause”, added Min as co-author, expanded coverage of Wiley Protocol and aromatase inhibitors, and added FAQ 06/01/19 Moved protocol specifics to appendix that can be published separately and changed dosing ratio from 8:3:1 to 6:3:1. Updated BG. 06/08/19 Released free version. Minor updates to text. 06/20/19 Updates to text and new reviews. 02/07/20 Minor updates to text, FAQ, and new reviews for the BG. 2 Chapter 1: Introduction .......................................................... 5 Chapter 2: The philosophy ...................................................... 9 Optimization vs Replacement ......................................... 9 What is “natural”? ........................................................ -

Collagen Peptides? Yet for Livestock, Although They Are Working on Them for Us
Danica Introducing Dr. Formulated Patrick CBD Probiotics Never Slowing Down! Two Premium Every Body Products in One Needs Collagen New Products with YOU in Mind $3.95 US $4.15 CAN YOURS FREE! FUEL FOR BODY & BRAIN Enjoy the convenience of on-the-go nutrition in powder form from Garden of Life. Our MCT Powder can be added to your favorite drinks for a boost of energy while our Butter Powder is perfect for cooking, baking or adding to side dishes. Keto convenience at your fingertips! †These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. healthEXTRAORDINARY VOLUME 38 • 2020 EDITOR Marilyn Gemino CONTRIBUTING EXPERT Dawn Thorpe Jarvis CONTRIBUTING WRITER Kelly Merritt Welcome to 2020 and as I say that, I feel like I’m talking about a sci-fi movie. PUBLISHING ART DIRECTOR Angela DiGloria-Coughter Not long ago, the year 2020 seemed so far off into the future. I’m here to tell you that there’s nothing fictional about some exciting new products we have in store SENIOR GRAPHICS MANAGER for you. But I will say, as always, there was science involved. Michael McGeary PHOTO DIRECTOR Innovation is always at the forefront of our Science and Innovation Team’s Day Tomko mind—and boy did they hit a home run here. We are so proud to introduce our Dr. Formulated CBD Probiotics line that uses a “capsule-in-a-capsule” technology, BRAND AMBASSADOR TEAM providing two premium products in one. Learn more about this amazing new line Carrie Bertram of products on page 22. -

Probiotics Research Paper
CALIFORNIA STATE UNIVERSITY SAN MARCOS PROJECT SIGNATURE PAGE PROJECT SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE MASTER OF BUSINESS ADMINISTRATION PROJECT TITLE: Probiotics Research Report AUTHORS: Michelle Carroll, Scott Morgans, Valerie Escalante PRESENTATION DATE: December 11, 2017 THE PROJECT HAS BEEN ACCEPTED BY THE PROJECT COMMITTEE IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF BUSINESS ADMINISTRATION. Name of Project Advisor Name of Second Reader PROJECT SECOND READER DATE Running head: PROBIOTICS RESEARCH REPORT Probiotics Research Report Michelle Carroll Valerie Escalante Scott Morgans California State University, San Marcos Author Note This report was prepared to satisfy Nicolas & Tomasevic Law Firms’s request for information surrounding the claims made by Probiotic companies in the United States. PROBIOTICS RESEARCH REPORT 2 Abstract The probiotics industry is a booming industry, expecting to grow an additional 38% by the year 2021. Under the Food and Drug Administration regulations the majority of probiotics are marketed as dietary supplements. This categorization of dietary supplements limits companies in pursuit of claims to the labeling of their products. Probiotic companies market their products for various health reasons, mainly digestive issues. In 2009, The Dannon Company became one of the first manufacturers to endure a class action lawsuit due to its claims being made on their probiotic yogurt packaging. This suit was then followed by other lawsuits filed against other large manufacturers. These types of lawsuits have lent a hand to manufacturers becoming more cautious about their claims and adding precautions to their labels. PROBIOTICS Research Report PREPARED BY Michelle Carroll Valerie Escalante Scott Morgans PROBIOTICS RESEARCH REPORT 3 The human body is covered inside and out with both harmful and beneficial bacteria. -

Citric Acid Is Widely Used in Food Processing
National Organic Standards Board | October 2019| Proposals & Discussion Documents National Organic Standards Board Meeting Doubletree Hotel & Suites Pittsburgh City Center | One Bigelow Square Pittsburgh, Pennsylvania | Pennsylvania Ballroom October 23-25, 2019 Title Page Handling Subcommittee (HS) | Asa Bradman, Chairperson 2021 Handling Sunset Reviews: §205.605 & §205.606 1 Crops Subcommittee (CS) | Steve Ela, Chairperson Proposal: Fatty Alcohol (C6, C8, C10, C12 Naturally Derived) - petitioned 67 Proposal: Potassium hypochlorite - petitioned 73 2021 Crops Sunset Reviews: §205.601 & §205.602 77 Discussion Document: Paper (Plant pots and other crop production aids) - petitioned 101 Materials Subcommittee (MS) | Emily Oakley, Chairperson Proposal: Excluded Methods: Induced mutagenesis and embryo transfer in livestock 109 Proposal: Genetic Integrity Transparency of Seed Grown on Organic Land 115 Proposal: NOSB Research Priorities 2019 121 Discussion Document: Marine materials in organic crop production 131 Policy Development Subcommittee (PDS) | Rick Greenwood, Chairperson Proposal: Updates to the policy & procedure manual (PPM) 149 Livestock Subcommittee (LS) | Sue Baird, Chairperson Proposal: Use of excluded method vaccines in organic livestock production 191 2021 Livestock Sunset Reviews: §205.603 201 Discussion Document: Fenbendazole - petitioned 227 PLEASE NOTE: Discussion documents, proposals, reports and/or other documents prepared by the National Organic Standards Board, including its subcommittees and task forces, represent the -

Food Forensics in Class Action Litigation: the Race Between Pleading Standards and Technology
Tulsa Law Review Volume 52 Issue 2 Article 19 Winter 2017 Food Forensics in Class Action Litigation: The Race Between Pleading Standards and Technology Jeff Lingwall Follow this and additional works at: https://digitalcommons.law.utulsa.edu/tlr Part of the Law Commons Recommended Citation Jeff Lingwall, Food Forensics in Class Action Litigation: The Race Between Pleading Standards and Technology, 52 Tulsa L. Rev. 213 (2017). Available at: https://digitalcommons.law.utulsa.edu/tlr/vol52/iss2/19 This Article is brought to you for free and open access by TU Law Digital Commons. It has been accepted for inclusion in Tulsa Law Review by an authorized editor of TU Law Digital Commons. For more information, please contact [email protected]. Lingwall: Food Forensics in Class Action Litigation: The Race Between Plead FOOD FORENSICS IN CLASS ACTION LITIGATION: THE RACE BETWEEN PLEADING STANDARDS AND TECHNOLOGY Jeff Lingwall* This Article examines the emerging use of “food forensics” to discover injury in class action litigation. Based on increased public interest in what goes inside food, plaintiffs are beginning to rely on statistical and chemical testing to verify label claims. The test results often spur producers to re-examine their products, but can also raise plausibility concerns under the veneer of science and deny consumers data they need to make informed decisions about food. Drawing on examples ranging from olive oil to multivitamins and canned octopus to pet food, I show how product testing in litigation represents a race between the resolving power of test results and slower- moving interpretation of pleading standards. -

2018 Membership Directory
2018 Membership Directory AHPA_directory_2018_v5.indd 1 6/27/18 1:33 PM TECHNOLOGY & TRANSPARENCY IN SUPPLY CHAIN SOURCING Offering a continuous supply of consistent quality ingredients from approved global factories of origin FACTORY-DIRECT PRICING QA/QC DOCS From the initial launch of the ingredientsonline. All documents and certificates are reviewed to com platform in 2015, we’ve increased our ensure they meet our standards prior to listing online factory partnerships by over 300%. them online. Each ingredient listing clearly displays Partnering directly with approved global all documentation in PDF form. Simply click to factories allows for factory-direct pricing which download any document, any time, 24/7. Watch for saves you on broker and distributor fees. Each third party test results including DNA testing on ingredient listing clearly displays tiered pricing select listings. for additional savings. U.S. INVENTORY FACTORY OF ORIGIN Every ingredient page clearly lists the real-time Knowing the original source of all ingredients is U.S. Inventory and you choose which warehouse to now mandatory to be FSMA/FSVP compliant. order from: CA, NJ or Miami. We’ve recently added All approved factories of origin are visibly our U.S. Direct Seller Program, giving you even transparent for every ingredient and at your more options for quality ingredients with clearly fingertips, 24/7. transparent U.S. inventory. ingredietnsonline.com offers ingredients from Canada, China, France, Germany, India, Indonesia, Italy, Korea, Pakistan, Romania, Thailand and the United States. We’re adding more factory partners and value- added ingredients weekly, so check the website often and watch for ingredients with research articles, clinical abstracts, branded and trade marked ingredients, DNA test results and ingredients with patents. -

Potential Application Areas for Fresenius Kabi´S Bonus Products
UPTEC X 19019 Examensarbete 30 hp Juni 2019 Potential application areas for Fresenius Kabi´s bonus products Elina Annala Jenny Karlsson Matilda Brink Abstract Potential application areas for Fresenius Kabi's bonus products Elina Annala, Jenny Karlsson, Matilda Brink Teknisk- naturvetenskaplig fakultet UTH-enheten The main purpose of the project was to investigate existing markets within the food- and cosmetic industry in order to determine a strategy Besöksadress: for market entrance with the bonus product of Fresenius Kabi. The Ångströmlaboratoriet Lägerhyddsvägen 1 original bonus product, P080, is a rest product from the process where Hus 4, Plan 0 phospholipids are extracted from egg yolk powder. P80 is a refined version of the P080 where egg yolk oil has been extracted, hence the Postadress: protein content in P80 is higher. The extracted egg yolk oil is the Box 536 751 21 Uppsala final portion of the bonus product. Thereby, the original bonus product can be divided into two refined fractions. This project investigates Telefon: the potential for each of these three substances to be a part of a 018 – 471 30 03 fictive product. Telefax: 018 – 471 30 00 By scanning the global protein market as well as the egg yolk protein- and egg oil market, seven potential product segments were discovered. Hemsida: These segments were further evaluated in order to bring out specific http://www.teknat.uu.se/student products that were potential candidates as products based on the bonus product. The analysis resulted in two fictive products based on P080; high value nutrition bars and snacks for seniors, one based on P80; protein powder, and one based on egg yolk oil; dietary supplement.