Food Forensics in Class Action Litigation: the Race Between Pleading Standards and Technology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

USCA Case #20-1145 Document #1881108 Filed: 01/21/2021 Page 1 of 43
USCA Case #20-1145 Document #1881108 Filed: 01/21/2021 Page 1 of 43 ORAL ARGUMENT NOT YET SCHEDULED UNITED STATES COURT OF APPEALS FOR THE DISTRICT OF COLUMBIA CIRCUIT COMPETITIVE ENTERPRISE INSTITUTE, et al., Petitioners, v. No. 20-1145 (consolidated with NATIONAL HIGHWAY TRAFFIC Nos. 20-1167, 20-1168, 20-1169, SAFETY ADMINISTRATION, et al., 20-1173, 20-1174, 20-1176, 20- 1177, 20-1230) Respondents, ALLIANCE FOR AUTOMOTIVE INNOVATION, et al. Intervenors for Respondents. BRIEF OF AMICUS CURIAE CONSUMER REPORTS IN SUPPORT OF COORDINATING PETITIONERS George P. Slover Cale Jaffe Senior Policy Counsel Director, Environmental Law and Consumer Reports Community Engagement Clinic 1101 17th St., NW, Suite 500 University of Virginia School of Law Washington, DC 20036 580 Massie Rd. Tel: (202) 462-6262 Charlottesville, VA 22903 [email protected] Tel: (434) 924-4776 [email protected] Dated: January 21, 2021 Counsel of Record for Amicus Curiae Consumer Reports USCA Case #20-1145 Document #1881108 Filed: 01/21/2021 Page 2 of 43 CERTIFICATE AS TO PARTIES, RULINGS, AND RELATED CASES A. Parties and Amici Except for the following, all Petitioners, Respondents, Intervenors, and Amici Curiae appearing before this Court are as listed in the Briefs of Petitioners Center for Biological Diversity, et al., City and County of Denver, et al., and National Coalition for Advanced Transportation, et al. Amici Curiae: Consumer Reports; and The Coalition to Protect America’s National Parks, the National Parks Conservation Association, New Mexico Wilderness Alliance; and American Thoracic Society, American Lung Association, American Medical Association, Medical Society of the District of Columbia; and National League of Cities, U.S. -

Essays on Transparent IT Support for Asymmetric Client-Advisor Encounters: the Case of Swiss Investment Advisory Services
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2012 Essays on transparent IT support for asymmetric client-advisor encounters: the case of Swiss investment advisory services Nussbaumer, Philipp Abstract: Investment advisory encounters are strained by information, knowledge and interest asymme- tries between client and advisor. These are detrimental to advisory quality and client satisfaction, leading to an unfavorable client perception of investment advisory services. This situation is disadvantageous for both clients and financial service providers. Clients increasingly turn to other information sources and fail to reap advisory services’ potential benefits for their investment decisions; financial service providers fail to exploit personalized advisory services as one the most promising differentiation strategies against competitors and struggle with low client satisfaction and retention. This dissertation suggests a novel approach for these issues: addressing asymmetries in investment advisory encounters with transparent, shared IT artifacts. Hence, it is based on the following thesis: Shared collaborative IT artifacts are a fea- sible and useful means to improve transparency of investment advisory encounters and, thus, to increase client satisfaction. The dissertation supports this thesis along three research essays: Essay I provides an empirical investigation of the status quo of Swiss investment advisory services. It suggests that investment advisory -

Consumer Organizations and Promotion of Sustainable Energy Consumption: Comparative Product Quality Testing and Its Impact
Panel III, 04 – Wahnschafft/Huh Consumer Organizations and Promotion of Sustainable Energy Consumption: Comparative Product Quality Testing and its Impact Ralph WAHNSCHAFFT Energy Resources Section, United Nations Economic and Social Commission for Asia and Pacific (UN-ESCAP), Bangkok, Thailand Kwisun HUH Department of Environmental Science & Engineering, Hanguk University of Foreign Studies, Seoul, Republic of Korea 1 - SYNOPSIS This paper reports selected preliminary results of an ongoing survey on consumer organizations. It analyzes comparative product quality testing and its potential impact on promotion of energy efficiency. 2 - ABSTRACT The paper provides an overview on consumer organizations in OECD and high income developing countries, in particular with regard to their consumer information activities. It focuses on a review of comparative product testing of selected electrical home appliances and on test reports in consumer interest magazines. The paper analyzes the relative weights allocated to the different product quality criteria, including energy efficiency. Based on the above the paper reflects on the role that consumer organizations can play in the promotion of “green consumerism” and sustainable energy consumption, in particular with regard to the residential sector. 3 - INTRODUCTION 3.1. Background and objectives Advancing commercialization of societies has brought with it the formation of a growing number of local and national consumer interest groups. Today, Consumers International, the largest federation of consumer organizations and agencies worldwide, counts 243 member organizations in over 110 countries (1). Individual organizations greatly differ in their membership and organization, their sources of financing and government sponsorship, their work methods, activities and relative political influence. However, enhancement of consumer protection and better consumer information are overall common objectives that unite the “consumer movement”. -
Five Questions to Ask When Considering Health Supplements
Five Questions to Ask When Considering Health Supplements COMMENTS JANUARY 19, 2016 / by KATIE WORTH Tow Journalism Fellow, FRONTLINE/Columbia Journalism School Fellowships Compared to most drugs sold at pharmacies, health supplements are loosely regulated by government agencies. Law prohibits manufacturers from selling products that are adulterated or mislabeled, and they cannot claim to cure things they don’t. But there is little oversight or enforcement to ensure they comply. And unlike prescription drugs, which pass through a strict premarket approval process, the Food and Drug Administration does not evaluate a supplement’s contents or effectiveness before it hits the shelves. Even then, the agency has only a modest capacity to test the pills. The result is a more than $30 billion industry that is largely regulated by the honor system. Given this framework, there is little to guarantee that any vitamin, mineral, probiotic, sports supplement, herbal treatment, or other dietary supplement is safe, effective, or even contains what’s on its label. Last year, for example, an investigation by the New York Attorney General’s office found that several popular store-brand supplements at four major retailers — GNC, Target, Walgreens and Walmart — contained contaminants not listed among the labeled ingredients. Just 21 percent of them actually had the DNA of the plant species they purported to be vending. While there are no guarantees, there are steps consumers can take to improve the chances that their supplements contain what they claim to, in the labeled quantities, and that they may indeed have a health benefit. Here are five questions a consumer may want to ask when considering supplements. -

The Price Sensitivity of Health Plan Choice Among Retirees: Evidence from the German Social Health Insurance
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Wuppermann, Amelie; Bauhoff, Sebastian; Grabka, Markus Conference Paper The Price Sensitivity of Health Plan Choice among Retirees: Evidence from the German Social Health Insurance Beiträge zur Jahrestagung des Vereins für Socialpolitik 2014: Evidenzbasierte Wirtschaftspolitik - Session: Health II, No. B10-V3 Provided in Cooperation with: Verein für Socialpolitik / German Economic Association Suggested Citation: Wuppermann, Amelie; Bauhoff, Sebastian; Grabka, Markus (2014) : The Price Sensitivity of Health Plan Choice among Retirees: Evidence from the German Social Health Insurance, Beiträge zur Jahrestagung des Vereins für Socialpolitik 2014: Evidenzbasierte Wirtschaftspolitik - Session: Health II, No. B10-V3, ZBW - Deutsche Zentralbibliothek für Wirtschaftswissenschaften, Leibniz-Informationszentrum Wirtschaft, Kiel und Hamburg This Version is available at: http://hdl.handle.net/10419/100352 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. -

Gauging the Influence of the Consumers Unions of the World Views of a Consumer Economist
GAUGING THE INFLUENCE OF THE CONSUMERS UNIONS OF THE WORLD VIEWS OF A CONSUMER ECONOMIST E. Scott Maynes, Cornell Universityl ~~~~~~~~~~ABSTRACT.~~~~~~~~~~ 3. What vehicles for influence (e.g., Influence : " t he effects of actions, behavior, product testing, advocacy offices) and opinions on others." This paper explores does each employ? the various dimensions of t he influence of t he Consumers Unions of the world and proposes My next two questions deal with what we need to measurements of each. Dimensions explored know : include a selective, a nnotated bibliography of publications that deal with t h eir influence, 4 . What has been the short-run influence estimates of the growth of U. S. consumer of each CU on (1) its readers/users organizations, a listing of t he "vehicles" for and (2) the public in i ts country? influence employed by Consumers Unions of U. S .A. (e.g., Consumer Reports), a model t hat depicts 5 . What has been the long-run influence the stages of influence t hrough which consumers of each CU on (1) its readers/users pass in the short - run (aware of it, approve of and (2) the public in i ts country? it, etc.) , and a benefit/cost model of the long run i nfluence of the Consumers Unions (e.g. , My paper consists primarily of five tables better choices . l onger life. etc.). which, suitably filled in, will answer these questions. The main publications of t he t hree largest Consumers Uni ons--Consumer s Unions of U.S .A. For completeness, I list four additional (hereafter CU), Consumers' Association of t he questions, worthy of consideration, that are United Kingdom (CA), and Sti ftung Warentest of excused for lack of space : Wes t Germany (SW)--are purchased by 2% to 5% of households in their countries and are cons ulte d 6. -

Why Third-Party GMP Certification Should Not Be Optional for Dietary Supplement Firms
Why Third-Party GMP Certification Should Not Be Optional for Dietary Supplement Firms By Scott Ravech Nutitional Outlook November 20, 2014 Quality took on a whole new meaning for me when I joined Deerland Enzymes as CEO in 2008. During my first tour of our manufacturing facility, I noticed that one of the enzyme products we produced was in fact the exact product that a naturopath had suggested for one of my sons. This personalized and drove home to me the seriousness of the responsibility that we as industry members have to ensure that the products we manufacture are absolutely safe for consumption. Current good manufacturing practices (GMPs), which require that companies have proper controls for the manufacturing, packaging, labeling, and storing of dietary supplements, are a key facet of product safety. FDA first introduced GMP regulations for dietary supplements (21 CFR Part 111) in 2007, with the intent of industry-wide compliance by 2010. By now, all companies should be GMP compliant. The industry itself—as well as consumers—pushed for greater safety regulations following concerns about substandard dietary supplement manufacturing practices and labeling. Unfortunately, those concerns continue to have merit. In 2013, FDA reported that as many as 70% of the GMP inspections it performed on dietary supplement manufacturing facilities identified GMP noncompliances. Independent testing by such groups as Consumer Reports and ConsumerLab.com continues to find incorrect or misbranded supplement labels in the market and, in some cases, supplements that contain unacceptable levels of contaminants. As manufacturers, we need to take supplement safety seriously. It is our responsibility to provide, above all else, safe dietary supplements that consumers can take without fear of harm. -

Summary of Annual Report 2016
Annual Report 2016 coming together for change I’d like to thank our members for their support of Consumers International and indeed their efforts for consumers around the world. I look forward to our ongoing impact and collaboration. Bart Combée President Contents 01 From our President 02 From our Director General 04 About Consumers International 06 Achievements and performance 08 International advocacy and campaigning 10 Building a digital world consumers can trust 12 Making international trade work for consumers 14 Advancing excellence in global consumer protection 16 World Consumer Rights Day 18 Campaigns, communications and brand development 20 Fundraising and partnerships 22 Consumers International’s governance 24 Financial summary 26 Trustees responsibilities statement 27 Respective responsibilities of trustees and auditor 27 Opinion on financial statements 27 Opinions on other matters prescribed by the Companies Act 2006 From our President, Bart Combée Bart Combée became President at Consumers And we’ve seen many successes this year. International’s General Assembly in Brasilia on • Consumers International played a major role in 21 November 2015 and is serving a four-year term. the adoption of an ISO standard on mobile services Mr Combée is also the Chief Executive of and new international guidelines to prevent the Consumentenbond in the Netherlands. mis-selling of financial services which will help create a better environment for consumers. I am pleased to report that Consumers International has had an excellent year of delivery for its members • In March more than 90 Consumers International and for consumers. members in 60 countries worked together to mark World Consumer Rights Day 2016 on the theme of The updates to our governance, which members voted antibiotic resistance, raising awareness of the overuse for at our 2015 World Congress have greatly supported and misuse of antibiotics in the food supply chain. -

10 Top Picks APRIL 2021
10 Top Picks APRIL 2021 For the latest ratings and information, visit These are the most exceptional new cars on the road today. They all shine in CR’s tests and surveys, and each comes standard with key advanced safety features. BY JEFF S. BARTLETT ach year more way onto our list through outstanding Consumer Reports. “That means we than 250 models performance in more than 50 tests at care more about how your next car or compete for the CR’s Auto Test Center, as well as having SUV performs when it comes to safety, hearts, minds, solid marks for reliability and owner comfort, and fuel efficiency than how and driveways satisfaction in our member surveys. All quickly it can circle a race track.” of American car have also scored well in dynamic safety Each Top Pick has an Overall Score shoppers. At tests, such as our challenging accident that’s among the highest in its category. Consumer Reports, we rank the new maneuver, and, if tested, earned And it must come standard with models we buy and evaluate based passing grades in crash assessments forward collision warning (FCW) and on regimented performance tests and conducted by the federal government automatic emergency braking (AEB) survey results from our members. Here, and the insurance industry. with pedestrian detection—proven we highlight the best of the best in our “We put thousands of miles on features that prevent injuries and save annual Top Picks, standouts in popular each test car and evaluate them for lives by reducing collisions. price categories and types. -

Still Not Safe: New Recalls Underline Need for Strong Hazardous Product Legislation
STILL NOT SAFE: NEW RECALLS UNDERLINE NEED FOR STRONG HAZARDOUS PRODUCT LEGISLATION A report by Consumers Union, publisher of Consumer Reports May 15, 2008 In its 2007 fiscal year, the U.S. Consumer Product Safety Commission (CPSC) announced a record 473 product recalls as the marketplace was besieged by unsafe toys and other products. The recalls included more than 25 million toys, tainted with hazardous lead paint, harmful, tiny magnets, toxic chemicals, and other dangers. In response, on December 19, 2007, the House of Representatives passed the CPSC Modernization Act, which gave the CPSC expanded powers and funding to improve product safety. The Senate passed its own CPSC reform legislation in early March. Members of the House and Senate are meeting now to hammer out a final agreement on a bill that can be endorsed by both houses and go to the President. It is essential that they combine the best consumer protection provisions in each bill. Much is still at stake. In just the first four months of 2008, the CPSC has recalled almost 10 million more consumer products. More than half of these, almost 6 million, were children’s products—toys, clothing, pacifiers, bicycles. More than 1.3 million of the children’s products were recalled because they contained dangerous levels of lead. And as in the past, the bulk of recalled products were imported from China—some 87 percent. Recalls, however, are not the solution; they only catch dangerous products after they have entered our stores, homes, and toy boxes. The real solution involves making certain that manufacturers test their products before they get to the market to ensure that they are safe for consumers, and penalizing those who do not comply with more stringent safety rules. -

Adverse Event Reporting System for Dietary Supplements: an Inadequate Safety Valve (OEI-01- 00-00180; 04/01)
Department of Health and Human Services OFFICE OF INSPECTOR GENERAL Adverse Event Reporting For Dietary Supplements An Inadequate Safety Valve APRIL 2001 OEI-01-00-00180 OFFICE OF INSPECTOR GENERAL The mission of the Office of Inspector General (OIG), as mandated by Public Law 95-452, is to protect the integrity of the Department of Health and Human Services programs as well as the health and welfare of beneficiaries served by them. This statutory mission is carried out through a nationwide program of audits, investigations, inspections, sanctions, and fraud alerts. The Inspector General informs the Secretary of program and management problems and recommends legislative, regulatory, and operational approaches to correct them. Office of Evaluation and Inspections The Office of Evaluation and Inspections (OEI) is one of several components of the Office of Inspector General. It conducts short-term management and program evaluations (called inspections) that focus on issues of concern to the Department, the Congress, and the public. The inspection reports provide findings and recommendations on the efficiency, vulnerability, and effectiveness of departmental programs. OEI's Boston Regional Office prepared this report under the direction of Mark R. Yessian, Ph.D., Regional Inspector General and Joyce M. Greenleaf, M.B.A., Assistant Regional Inspector General. Principal OEI staff included: BOSTON HEADQUARTERS Laura C. McBride, Lead Analyst Elise Stein, Program Specialist Aimee L. Kasenga, Program Analyst Joseph Rutherford, Program Specialist Nancy L. London, Program Analyst Nicola Y. Pinson, Program Analyst To obtain copies of this report, please call the Boston Regional Office at (617) 565-1050. Reports are also available on the World Wide Web at our home page address: http://www.dhhs.gov/oig/oei EXECUTIVE SUMMARY PURPOSE To assess the effectiveness of the Food and Drug Administration’s (FDA) adverse event reporting system for dietary supplements in protecting the American consumer. -
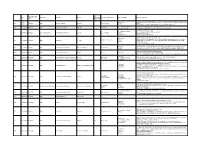
Category of Supplement Types of Claims Examples of Claims (2007) Enforceme Nt? •Germs Are Everywhere
Referred to Gov Date of Decision Type Challenger Advertiser Product Agency for Category of Supplement Types of Claims Examples of Claims (2007) Enforceme nt? •Germs are everywhere. Take Airborne to boost your immune system, fight viruses and help you stay Case •Performance 4648 3/26/2007 NAD Airborne Health Inc. Airborne Immune Health healthy. Report •Implied •Take Airborne. The immune boosting tablet that helps your body fight germs. •Clinically Proven/Shown •CH-Alpha is scientifically proven to promote joint health. 4652 Case Report 4/10/2007 NAD Gelita Health Products CH-Alpha Joint Health •Preventative Health •After just two to three months, you will regain the freedom of flexibility. •94% faster recovery [from colds] •Clinically Proven/Shown •Increased immune system resistance by 312% 4653 Case Report 4/10/2007 Proctor and Gamble Iovate Health Sciences Inc. Cold MD Immune Health •Speed •Clinically Proven results •Comparative •Doctor formulated and approved •Chromax helps your insulin function at its best. •Performance •It's an advanced, highly absorbable form of chromium that provides your body with the chromium it Blood Sugar Health •Insulin 4547 Compliance 4/11/2007 NAD Nutrition 21 Chromax needs to help promote healthy blood sugar, fight carbohydrate cravings and support your overall •Implied cardiovascular health. •Essential for optimum insulin health. •Exclusivity •One-A-Day Women's Multi-Vitamin is the only complete multi-vitamin with more calcium for strong 4672 Case Report 5/1/2007 NAD Bayer Consumer Healthcare One-A-Day Women's Multi-Vitamin •Implied bones, and now more vitamin D, which emerging research suggests may support breast cancer.