Program of Photonics and Its Applications to Medicine Professor T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UW Med Phys Program Overview
UW Medical Physics Graduate Program Orientation 2019 Edward F. Jackson, PhD Chair, Department of Medical Physics & Program Director [email protected] Department Overview • One of 10 Basic Science departments in UW School of Medicine and Public Health • 93 faculty, including emeritus, joint, affiliate, adjunct, volunteer, and honorary fellow appointments • Faculty at SMPH: • 24 tenured/tenure track (many with joint appointments) • 5 clinical health science (CHS) track • 1 clinical teaching track • 10 Emeritus professors (including past Provost and two previous dept chairs) • 2 Joint department appointments (in Radiology) • 26 Affiliates (in Radiology, DHO, Engineering, Medicine, Psychiatry) UW-Madison Medical Physics “West Campus” Health-Centric LOCI Physics & Math Wisconsin Institutes of Discovery Morgridge Institute for Research Engineering, CS, Statistics Locations of Key Resources WIMR Towers Pharmacy Waisman School Center Tower 2 Tower 1 Children’s Hospital Medical School Nursing (Health Sciences Learning Center – School HSLC) Ebling Library University Hospital & Clinics VA Hospital 1. Wisconsin Institutes of Medical Research (WIMR 1) 2. UW Carbone Comprehensive Cancer Center 3. UW Hospitals & Clinics 4. UW School of Medicine and Public Health (SMPH) 2 3 4 1 Personnel You Should Know • Chair and Program Director: Ed Jackson, PhD WIMR 1016 • Assistant to the Chair: Alyssa Mohr WIMR 1018 Scheduling appointments with chair; conference room scheduling; car/van • Graduate Committee Chair: Tomy Varghese WIMR 1159 Initial approval of warrants; -
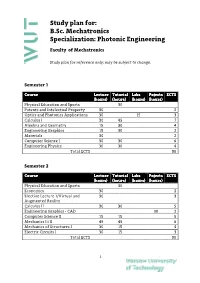
B.Sc. Mechatronics Specialization: Photonic Engineering
Study plan for: B.Sc. Mechatronics Specialization: Photonic Engineering Faculty of Mechatronics Study plan for reference only; may be subject to change. Semester 1 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Patents and Intelectual Property 30 2 Optics and Photonics Applications 30 15 3 Calculus I 30 45 7 Algebra and Geometry 15 30 4 Engineering Graphics 15 30 2 Materials 30 2 Computer Science I 30 30 6 Engineering Physics 30 30 4 Total ECTS 30 Semester 2 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Economics 30 2 Elective Lecture 1/Virtual and 30 3 Augmented Reality Calculus II 30 30 5 Engineering Graphics ‐ CAD 30 2 Computer Science II 15 15 5 Mechanics I i II 45 45 6 Mechanics of Structures I 30 15 4 Electric Circuits I 30 15 3 Total ECTS 30 1 Study Plan for B.Sc. Mechatronics (Spec. Photonic Engineering) Semester 3 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Elective Lecture 2/Introduction to 30 3 MEMS Calculus III 15 30 6 Mechanics of Structures II 15 15 4 Manufacturing Technology I 30 4 Fine Machine Design I 15 30 3 Electric Circuits II 30 3 Basics of Automation and Control I 30 15 4 Total ECTS 31 Semester 4 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Foreign Language 60 4 Elective Lecture 3/Photographic 30 3 techniques in image acqusition Elective Lecture 4 30 3 /Enterpreneurship Optomechatronics 30 30 5 Electronics I 15 15 2 Electronics II 15 1 Fine Machine Design II 15 15 3 Manufacturing Technology 30 2 Metrology 30 30 4 Total ECTS 27 Semester 5 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Marketing 30 2 Elective Lecture 5/ Electric 30 2 2 Study Plan for B.Sc. -

Read About the Future of Packaging with Silicon Photonics
The future of packaging with silicon photonics By Deborah Patterson [Patterson Group]; Isabel De Sousa, Louis-Marie Achard [IBM Canada, Ltd.] t has been almost a decade Optics have traditionally been center design. Besides upgrading optical since the introduction of employed to transmit data over long cabling, links and other interconnections, I the iPhone, a device that so distances because light can carry the legacy data center, comprised of many successfully blended sleek hardware considerably more information off-the-shelf components, is in the process with an intuitive user interface that it content (bits) at faster speeds. Optical of a complete overhaul that is leading to effectively jump-started a global shift in transmission becomes more energy significant growth and change in how the way we now communicate, socialize, efficient as compared to electronic transmit, receive, and switching functions manage our lives and fundamentally alternatives when the transmission are handled, especially in terms of next- interact. Today, smartphones and countless length and bandwidth increase. As the generation Ethernet speeds. In addition, other devices allow us to capture, create need for higher data transfer speeds at as 5G ramps, high-speed interconnect and communicate enormous amounts of greater baud rate and lower power levels between data centers and small cells will content. The explosion in data, storage intensifies, the trend is for optics to also come into play. These roadmaps and information distribution is driving move closer to the die. Optoelectronic will fuel multi-fiber waveguide-to-chip extraordinary growth in internet traffic interconnect is now being designed interconnect solutions, laser development, and cloud services. -

Merging Photonics and Artificial Intelligence at the Nanoscale
Intelligent Nanophotonics: Merging Photonics and Artificial Intelligence at the Nanoscale Kan Yao1,2, Rohit Unni2 and Yuebing Zheng1,2,* 1Department of Mechanical Engineering, The University of Texas at Austin, Austin, Texas 78712, USA 2Texas Materials Institute, The University of Texas at Austin, Austin, Texas 78712, USA *Corresponding author: [email protected] Abstract: Nanophotonics has been an active research field over the past two decades, triggered by the rising interests in exploring new physics and technologies with light at the nanoscale. As the demands of performance and integration level keep increasing, the design and optimization of nanophotonic devices become computationally expensive and time-inefficient. Advanced computational methods and artificial intelligence, especially its subfield of machine learning, have led to revolutionary development in many applications, such as web searches, computer vision, and speech/image recognition. The complex models and algorithms help to exploit the enormous parameter space in a highly efficient way. In this review, we summarize the recent advances on the emerging field where nanophotonics and machine learning blend. We provide an overview of different computational methods, with the focus on deep learning, for the nanophotonic inverse design. The implementation of deep neural networks with photonic platforms is also discussed. This review aims at sketching an illustration of the nanophotonic design with machine learning and giving a perspective on the future tasks. Keywords: deep learning; (nano)photonic neural networks; inverse design; optimization. 1. Introduction Nanophotonics studies light and its interactions with matters at the nanoscale [1]. Over the past decades, it has received rapidly growing interest and become an active research field that involves both fundamental studies and numerous applications [2,3]. -

Undergraduate Course on Biomedical Imaging at a Liberal Arts College
Undergraduate Course on Biomedical Imaging at a Liberal Arts College Michael E. Dursta aMiddlebury College, Middlebury, VT 05753, USA ABSTRACT This paper presents an intermediate-level undergraduate course on the physical principles of biomedical optics and imaging. Through in-class labs, Mathematica simulations, field trips, and group presentations, students learn about fundamental imaging concepts in optical microscopes. After developing an understanding of the role of the Fourier transform in image formation, the course shifts to non-optical imaging, including x-ray computed tomography, ultrasound, and magnetic resonance imaging. The significance of this course is its hands- on nature, and this paper offers examples of laboratory exercises and simulations to promote active learning in the classroom. Keywords: biomedical optics, imaging, undergraduate course, lab exercises, Mathematica simulations 1. INTRODUCTION In this paper, I present an intermediate-level physics course on biomedical imaging, with the goal of sharing resources to aid in the development of similar undergraduate optics courses.1{6 I introduced this course for three reasons: to provide an interdisciplinary physics course to support a liberal arts education, to attract students who are underrepresented in physics to the major, and to bring my research on biomedical optics into the classroom. Beyond the students' interest in the subject matter, this course works well because the physical phenomena are both visual and hands-on in nature, although simulations and a field trip to a hospital radiology department are required for most non-optical imaging techniques. Beginning with the study of geometric optics, students explore the concepts of image formation by building a microscope from scratch. -

Medical Physics
FACULTY OF SCIENCE MEDICAL PHYSICS Clinical Medical and Health Physics is an exciting and expanding field that applies our fundamental knowledge of physics to the prevention, diagnosis and treatment of a variety of human conditions. Ultrasound, Magnetic Resonance, Computed Tomography, Nuclear Medicine, X-rays, Radiation Therapy, are all branches of medical physics in which continued research is being conducted by a very large group of dedicated researchers consisting of highly qualified physicists, engineers and radiologists. The program at UWinnipeg leads to a Bachelor of Science degree (4-year Honours) and provides excellent preparation for entry into a graduate program, such as the two-year MSc program at the University of Manitoba through the Division of Medical Physics at CancerCare Manitoba. (Currently, the recommended training for medical physicists is a degree at the graduate level.) Many graduates go on to become members of the Canadian College of Physicists in Medicine (CCPM) by passing written examinations. CCPM certification is becoming widely accepted in Canada and other countries and is often required at senior levels in medical physics. Also, please see other related fact sheets: “Physics” and “Computational Physics” SAMPLE CAREERS Most medical physicists work in hospital diagnostic imaging departments, cancer treatment facilities, or hospital-based research establishments, while others work in universities, government, and industry. Here are a few examples of specific positions: clinical medical physicist; radiation safety officer for medical radioisotope facilities; radiotherapy physicist who helps design/construct radiotherapy treatment equipment or who researches the use of heat and lasers in cancer treatment. SAMPLE COURSES Human Anatomy and Physiology: This course deals with the biological study of the human organism; microscopic and gross anatomy; cellular and general physiology, and human genetics. -

Experimental Photonics Multiple Post-Doctoral Positions Experimental Expertise in Any One of the Following Topics/Areas Is Highly Desired
Experimental Photonics Multiple Post-Doctoral Positions Experimental Expertise in any one of the following topics/areas is highly desired . Single photon level measurements , quantum communications . Computational imaging, super-resolution imaging, biomedical imaging . Quantum dots, 2D materials, quantum devices, quantum transport . Single molecule spectroscopy/imaging . Fluorescence microscopy . Optical manipulation of spin , ODMR, Magnetometry, NV centers . Nanofabication (Metasurfaces, plasmonics,silicon photonics) . Streak camera or time-correlated single photon counting experiments . Ultrafast spectroscopy, pump-probe measurements . Single nanoparticle/nanoantenna experiments . Coupling of single quantum emitters to nanophotonic structures . Cold atoms and quantum optics . Infrared spectroscopy, thermal emission measurements Please send your full CV and three representative publications to: [email protected] Prof. Zubin Jacob Birck Nanotechnology Center School of Electrical and Computer Engineering Purdue University, U.S.A. www.electrodynamics.org Zubin Jacob Research Group: Purdue University www.electrodynamics.org About the group Google Scholar Page: https://scholar.google.ca/citations?user=8FXvN_EAAAAJ&hl=en Main Research Areas: Casimir forces, quantum nanophotonics, plasmonics, metamaterials, Vacuum fluctuations, open quantum systems Weblink: www.electrodynamics.org Theory and Experiment Twitter: twitter.com/zjacob_group • Opportunity to closely interact with theorists and experimentalists within the group • Opportunity to travel -

Photonics Engineer
Photonics Engineer Antelope company Antelope DX develops a point-of-need diagnostic platform that allows consumers and healthcare professionals to have on-the-spot access to key health parameters. The Antelope technology aims to offer clinical lab performance with the ease-of-use of a pregnancy test at a consumer price tag. The platform is based on an innovative lab-on-chip technology that can perform a sensitive test on any bodily fluid, without requiring complex user operations or sample preparation. Role The Antelope Photonics Engineer is responsible for the design & development of the silicon photonic chip, located inside the Antelope consumable. He/she will also contribute largely to the optics and photonics aspects of associated hardware such as the Antelope reader. He/she will need to perform these product developments in a way that is compatible to IVD industry standards, including the generation of associated documentation. Responsibilities and duties • Photonics design & optimization of the sensing circuits. • Set up an optical/photonic system model to better predict and understand deviations from the norm by e.g. manufacturing tolerances. • Setting up characterisation, verification and QC equipment and methodologies for the photonic wafers & chips. • Support the design of the optical components of the read-out system. • Support the developmentt of the algorithmic framework that processes the optical signals to a diagnostic answer. • Support the development of R&D tools & methodologies from a system perspective to increase R&D efficiency, throughput and data generation. • Support the improvement of the R&D experimental setups, used to generate assay results. • Setting up testing and verification planning. -

Illuminating the History and Expanding Photonics Education
Illuminating the History and Expanding Photonics Education An Interactive Qualifying Project submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE in partial fulfilment of the requirements for the degree of Bachelor of Science by Nicholas Marshall Brandon McLaughlin Date: 2nd June 2020 Report Submitted to: Worcester Polytechnic Institute Quinsigamond Community College Professor Douglas Petkie Worcester Polytechnic Institute This report represents work of WPI undergraduate students submitted to the faculty as evidence of a degree requirement. WPI routinely publishes these reports on its web site without editorial or peer review. For more information about the projects program at WPI, see http://www.wpi.edu/Academics/Projects. Abstract Photonics today is on the cusp of revolutionizing computing, just as it has already revolutionized communication, and becoming to this century what electricity was to the last (Sala, 2016). As the manifestation of mankind's millenia-spanning obsession with light, photonics evolved from optics, which itself developed over the long course of human history. That development has accelerated in the last several centuries, and today optics and photonics act as enablers for a variety of fields from biology to communication. Even so, most people don’t know just how essential optics and photonics are, and today those fields face a major staffing shortage. Most people don’t even know the basic principles of light’s behavior, with few formal education programs that focus on optics and photonics. In order to combat this, various initiatives have strived to drum up more interest in optics and photonics, with several focusing on pre-college age groups in order to get students involved sooner. -

Smph-Medical-Physics-Chair-Search
THE SEARCH FOR THE CHAIR, DEPARTMENT OF MEDICAL PHYSICS Madison, Wisconsin The University of Wisconsin School of Medicine and Public Health invites applications and nominations for the position of chair of the Department of Medical Physics. The Opportunity The Department of Medical Physics (DMP) is a leader in conducting groundbreaking basic and translational research and education leading to new applications of physics in medicine and biology. The department’s mission is to visualize solutions for accurate diagnosis and treatment by focusing on medical imaging, radiotherapy, biomagnetism, and radiation metrology yields, tools, and methods. In doing so, DMP benefits patients in our community and worldwide. DMP has the largest medical physics graduate program in North America. More than 30 years ago, it was the first to be accredited by the Commission on Accreditation of Medical Physics Education Programs (CAMPEP). It also offers a 24-month residency in medical physics. Through training and collaboration with clinicians in fields including radiation oncology, radiology, cardiology, and neurosurgery, members of the department assure excellent patient care with advanced diagnostic and therapeutic equipment and techniques. A key and distinguishing feature of DMP is its institutional setting within a top-tier research institution and the nation’s first School of Medicine and Public Health (SMPH). With longstanding, close collaborations with departments across SMPH and the College of Engineering, as well as with industry collaborators, DMP is at the forefront of building partnerships to advance education, research, and patient outcomes. The department is resource-rich and features state-of-the-art imaging systems in all clinical imaging modalities that are devoted to research. -

The Role of Nanostructures in Integrated Photonics
The role of Nanostructures in Integrated Photonics James S. Harris Department of Electrical Engineering Stanford University 3rd U.S.-Korea Forum on Nanotechnology Seoul, Korea April 3 & 4, 2006 U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 1 1993 Photonic Integrated Circuit Soref, Proc. IEEE, 1687 (1993) z Waveguide architecture with butt coupled fibers and edge emitting lasers z Hybrid bonding (non-monolithic) of different structures z Mostly III-V devices, very little electronics U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 2 First Photonic Crystal Device DBR (Distributed Bragg Reflector z Single longitudinal mode emission, z 20-40 quarter wavelength independent of temperature and current injection different index layers (~70 nm) z Circular beam pattern z One-dimensional photonic crystal z Vertical emission--2-D array U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 3 Dimensional Mismatch Between Optics and Electronics U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 4 Unique Photonic Crystal Functionality Electric Field Strength U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 5 Nanoscale Plasmonic Waveguides z 90° bends and splitters can be designed with 100% transmission from microwave to optical frequencies z Provides bridge between dimensions of electronics and photonics z Provides design flexibility for optoelectronic ICs U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 6 A New Si-Based Optical Modulator Quantum-confined Stark effect (QCSE) z Strongest high-speed optical modulation mechanism z Used today for high-speed, low power telecommunications optical modulators but in III-V semiconductors z QCSE in germanium quantum wells on silicon substrates z Fully compatible with CMOS fabrication z Surprises z works in “indirect gap” semiconductor actually better than in III-V z higher speed (100 GHz) possible Y. -

The (Pre-)History of Medical Physics
The (Pre-)History of Medical Physics (we’re older than you believe) Warning: This presentation contains multiple images of dead physicists. Viewer discretion is advised. Often things go back earlier than we think! Recently found in the pocket of a geek in Switzerland Recently found in a cave in Switzerland Where these the first medical physicists? Pierre and Marie Curie in their lab Or this guy? Henri Bequerel (Curies’ mentor) Early isotope radiograph (1896) Or perhaps Roentgen (1895)? Or the real discoverer of x-rays (1857)? Or the real discoverer of x-rays (1857)? Claude Felix Abel Niepce de Saint-Victor You MUST read this book! How many can you name? How many can you name? Borelli Pelletan Fick Draper Bird Halle Where it all began - Padua The first medical physicist? Sanctorius (1561 – 1636) (actually, he was a medic) The first real medical physicist? Giovanni Borelli (1608 – 1679) Professor of Mathematics Pisa “I undertook this work … to enlist anatomy into physics and mathematics no less than astronomy” Iatrophysics Borelli also considered • Muscle contraction • “nervous juice” (nerve conduction) • Cardiovascular haemodynamics • Body heat • Respiration • Kidney and liver function Boyle and Hooke (about 1660) • Respiration • Animal experiments • Purpose of breathing was to bring air into the lungs so that air could interact with the blood Hooke’s Microscope Daniel Bernoulli (1700 – 1782) Medicine and mathematics • Respiration • Optics of vision • Muscle action Euler (1707 – 1783) “On the Blood Flow in the Arteries” 푑푠 푑(푣푠) + =0 푑푡