Photonics Engineer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
B.Sc. Mechatronics Specialization: Photonic Engineering
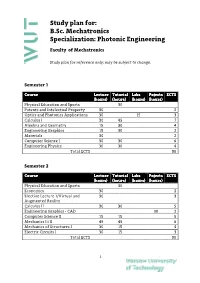
Study plan for: B.Sc. Mechatronics Specialization: Photonic Engineering Faculty of Mechatronics Study plan for reference only; may be subject to change. Semester 1 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Patents and Intelectual Property 30 2 Optics and Photonics Applications 30 15 3 Calculus I 30 45 7 Algebra and Geometry 15 30 4 Engineering Graphics 15 30 2 Materials 30 2 Computer Science I 30 30 6 Engineering Physics 30 30 4 Total ECTS 30 Semester 2 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Economics 30 2 Elective Lecture 1/Virtual and 30 3 Augmented Reality Calculus II 30 30 5 Engineering Graphics ‐ CAD 30 2 Computer Science II 15 15 5 Mechanics I i II 45 45 6 Mechanics of Structures I 30 15 4 Electric Circuits I 30 15 3 Total ECTS 30 1 Study Plan for B.Sc. Mechatronics (Spec. Photonic Engineering) Semester 3 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Elective Lecture 2/Introduction to 30 3 MEMS Calculus III 15 30 6 Mechanics of Structures II 15 15 4 Manufacturing Technology I 30 4 Fine Machine Design I 15 30 3 Electric Circuits II 30 3 Basics of Automation and Control I 30 15 4 Total ECTS 31 Semester 4 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Foreign Language 60 4 Elective Lecture 3/Photographic 30 3 techniques in image acqusition Elective Lecture 4 30 3 /Enterpreneurship Optomechatronics 30 30 5 Electronics I 15 15 2 Electronics II 15 1 Fine Machine Design II 15 15 3 Manufacturing Technology 30 2 Metrology 30 30 4 Total ECTS 27 Semester 5 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Marketing 30 2 Elective Lecture 5/ Electric 30 2 2 Study Plan for B.Sc. -

Read About the Future of Packaging with Silicon Photonics
The future of packaging with silicon photonics By Deborah Patterson [Patterson Group]; Isabel De Sousa, Louis-Marie Achard [IBM Canada, Ltd.] t has been almost a decade Optics have traditionally been center design. Besides upgrading optical since the introduction of employed to transmit data over long cabling, links and other interconnections, I the iPhone, a device that so distances because light can carry the legacy data center, comprised of many successfully blended sleek hardware considerably more information off-the-shelf components, is in the process with an intuitive user interface that it content (bits) at faster speeds. Optical of a complete overhaul that is leading to effectively jump-started a global shift in transmission becomes more energy significant growth and change in how the way we now communicate, socialize, efficient as compared to electronic transmit, receive, and switching functions manage our lives and fundamentally alternatives when the transmission are handled, especially in terms of next- interact. Today, smartphones and countless length and bandwidth increase. As the generation Ethernet speeds. In addition, other devices allow us to capture, create need for higher data transfer speeds at as 5G ramps, high-speed interconnect and communicate enormous amounts of greater baud rate and lower power levels between data centers and small cells will content. The explosion in data, storage intensifies, the trend is for optics to also come into play. These roadmaps and information distribution is driving move closer to the die. Optoelectronic will fuel multi-fiber waveguide-to-chip extraordinary growth in internet traffic interconnect is now being designed interconnect solutions, laser development, and cloud services. -

Merging Photonics and Artificial Intelligence at the Nanoscale
Intelligent Nanophotonics: Merging Photonics and Artificial Intelligence at the Nanoscale Kan Yao1,2, Rohit Unni2 and Yuebing Zheng1,2,* 1Department of Mechanical Engineering, The University of Texas at Austin, Austin, Texas 78712, USA 2Texas Materials Institute, The University of Texas at Austin, Austin, Texas 78712, USA *Corresponding author: [email protected] Abstract: Nanophotonics has been an active research field over the past two decades, triggered by the rising interests in exploring new physics and technologies with light at the nanoscale. As the demands of performance and integration level keep increasing, the design and optimization of nanophotonic devices become computationally expensive and time-inefficient. Advanced computational methods and artificial intelligence, especially its subfield of machine learning, have led to revolutionary development in many applications, such as web searches, computer vision, and speech/image recognition. The complex models and algorithms help to exploit the enormous parameter space in a highly efficient way. In this review, we summarize the recent advances on the emerging field where nanophotonics and machine learning blend. We provide an overview of different computational methods, with the focus on deep learning, for the nanophotonic inverse design. The implementation of deep neural networks with photonic platforms is also discussed. This review aims at sketching an illustration of the nanophotonic design with machine learning and giving a perspective on the future tasks. Keywords: deep learning; (nano)photonic neural networks; inverse design; optimization. 1. Introduction Nanophotonics studies light and its interactions with matters at the nanoscale [1]. Over the past decades, it has received rapidly growing interest and become an active research field that involves both fundamental studies and numerous applications [2,3]. -

Experimental Photonics Multiple Post-Doctoral Positions Experimental Expertise in Any One of the Following Topics/Areas Is Highly Desired
Experimental Photonics Multiple Post-Doctoral Positions Experimental Expertise in any one of the following topics/areas is highly desired . Single photon level measurements , quantum communications . Computational imaging, super-resolution imaging, biomedical imaging . Quantum dots, 2D materials, quantum devices, quantum transport . Single molecule spectroscopy/imaging . Fluorescence microscopy . Optical manipulation of spin , ODMR, Magnetometry, NV centers . Nanofabication (Metasurfaces, plasmonics,silicon photonics) . Streak camera or time-correlated single photon counting experiments . Ultrafast spectroscopy, pump-probe measurements . Single nanoparticle/nanoantenna experiments . Coupling of single quantum emitters to nanophotonic structures . Cold atoms and quantum optics . Infrared spectroscopy, thermal emission measurements Please send your full CV and three representative publications to: [email protected] Prof. Zubin Jacob Birck Nanotechnology Center School of Electrical and Computer Engineering Purdue University, U.S.A. www.electrodynamics.org Zubin Jacob Research Group: Purdue University www.electrodynamics.org About the group Google Scholar Page: https://scholar.google.ca/citations?user=8FXvN_EAAAAJ&hl=en Main Research Areas: Casimir forces, quantum nanophotonics, plasmonics, metamaterials, Vacuum fluctuations, open quantum systems Weblink: www.electrodynamics.org Theory and Experiment Twitter: twitter.com/zjacob_group • Opportunity to closely interact with theorists and experimentalists within the group • Opportunity to travel -

Illuminating the History and Expanding Photonics Education
Illuminating the History and Expanding Photonics Education An Interactive Qualifying Project submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE in partial fulfilment of the requirements for the degree of Bachelor of Science by Nicholas Marshall Brandon McLaughlin Date: 2nd June 2020 Report Submitted to: Worcester Polytechnic Institute Quinsigamond Community College Professor Douglas Petkie Worcester Polytechnic Institute This report represents work of WPI undergraduate students submitted to the faculty as evidence of a degree requirement. WPI routinely publishes these reports on its web site without editorial or peer review. For more information about the projects program at WPI, see http://www.wpi.edu/Academics/Projects. Abstract Photonics today is on the cusp of revolutionizing computing, just as it has already revolutionized communication, and becoming to this century what electricity was to the last (Sala, 2016). As the manifestation of mankind's millenia-spanning obsession with light, photonics evolved from optics, which itself developed over the long course of human history. That development has accelerated in the last several centuries, and today optics and photonics act as enablers for a variety of fields from biology to communication. Even so, most people don’t know just how essential optics and photonics are, and today those fields face a major staffing shortage. Most people don’t even know the basic principles of light’s behavior, with few formal education programs that focus on optics and photonics. In order to combat this, various initiatives have strived to drum up more interest in optics and photonics, with several focusing on pre-college age groups in order to get students involved sooner. -

The Role of Nanostructures in Integrated Photonics
The role of Nanostructures in Integrated Photonics James S. Harris Department of Electrical Engineering Stanford University 3rd U.S.-Korea Forum on Nanotechnology Seoul, Korea April 3 & 4, 2006 U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 1 1993 Photonic Integrated Circuit Soref, Proc. IEEE, 1687 (1993) z Waveguide architecture with butt coupled fibers and edge emitting lasers z Hybrid bonding (non-monolithic) of different structures z Mostly III-V devices, very little electronics U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 2 First Photonic Crystal Device DBR (Distributed Bragg Reflector z Single longitudinal mode emission, z 20-40 quarter wavelength independent of temperature and current injection different index layers (~70 nm) z Circular beam pattern z One-dimensional photonic crystal z Vertical emission--2-D array U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 3 Dimensional Mismatch Between Optics and Electronics U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 4 Unique Photonic Crystal Functionality Electric Field Strength U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 5 Nanoscale Plasmonic Waveguides z 90° bends and splitters can be designed with 100% transmission from microwave to optical frequencies z Provides bridge between dimensions of electronics and photonics z Provides design flexibility for optoelectronic ICs U.S.-Korea Forum on Nanotechnology-Korea-4/3/06 JSH 6 A New Si-Based Optical Modulator Quantum-confined Stark effect (QCSE) z Strongest high-speed optical modulation mechanism z Used today for high-speed, low power telecommunications optical modulators but in III-V semiconductors z QCSE in germanium quantum wells on silicon substrates z Fully compatible with CMOS fabrication z Surprises z works in “indirect gap” semiconductor actually better than in III-V z higher speed (100 GHz) possible Y. -
Photonics and Electro-Optical Engineering
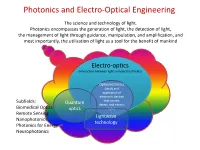
Photonics and Electro-Optical Engineering The science and technology of light. Photonics encompasses the generation of light, the detection of light, the management of light through guidance, manipulation, and amplification, and most importantly, the utilization of light as a tool for the benefit of mankind Electro-optics (interaction between light and electrical fields) Optoelectronics (study and application of electronic devices Subfields: that source, Quantum detect, and control Biomedical Optics optics light) Remote Sensing Lightwave Nanophotonics technology Photonics for Energy Neurophotonics Photonics and Electro-Optical Engineering Photonics is an enabling technology1 with general-purpose characteristics applications in : • Communications, information processing and data storage • Health and medicine • Energy • Defense and homeland security • Advanced manufacturing • Advanced metrology 1 enabling general-purpose technology – technology that advances foster innovations across a broad spectrum of applications in a diverse array of economic sectors. Other examples of general-purpose enabling technologies are the transistor and the integrated circuit. Photonics engineering –an enabling technology Not always seen but it is ubiquitous . Consider for example using a smartphone to perform an Internet search. Where is the optics? Efficient Flash light Smart Camera High resolution display cell tower Microprocessor fabricated using optical lithographic techniques fiber optic network Chips inspected with photonics techniques more than 1 million lasers involved in the data signaling Data center 20km of fibers Photonics and Electro-Optical Engineering in the 21st century The term of photonics was coined in analogy with electronics: Electronics involves the control of electric-charge flow (in vacuum or matter); Photonics involves the control of photons (in free space and matter) It is expected that the 21st century will depend as much on photonics as the 20th century depended on electronics1. -

Job Profile Photonics Engineer
Job Profile Photonics Engineer Job Details Job Title: Photonics Engineer Reports to: Prof William Gillin Salary: £40K-£45k p.a. dependent on experience Appointment period: Permanent Full Time Current Location: Mile End Campus, Queen Mary University of London Closing date Job Context Chromosol Ltd is a spin out from Queen Mary, University of London (QMUL) which will commercialise an organic sensitized rare earth optical amplifier technology to enter the £3 bn optical transceiver and optical amplifier markets, part of the broader electronics sector. Professor William Gillin, of the School of Physics and Astronomy, has demonstrated an organic optical gain material which will extend existing fibre optic technology to operate on a much shorter scale, of the order of a metre between racks and servers in datacentres, and on the cm scale between silicon chips in devices. IP Group Plc, the UK’s largest early stage technology investor (www.ipgroupplc.com), have invested in Chromosol and, in addition, Chromosol has been awarded funding from Innovate UK. The early stage research will take place within QMUL’s laboratories, and specifically in the School of Physics and Astronomy on the Mile End Campus. Job Purpose The Photonics Engineer will work with Prof William Gillin the CTO of Chromosol Ltd and Dr Huanqing Ye, Senior Research Scientist at Chromosol Ltd. This role will be to develop and test a new class of photonic integrated circuits (PICs), primarily on a Silicon Nitride platform but moving on to a Silicon Photonics platform. The successful applicant will be responsible for the specification, simulation, design and testing of PICs to be manufactured by commercial suppliers. -

Engineering Photonics Solutions for COVID-19
Engineering photonics solutions for COVID-19 Cite as: APL Photonics 5, 090901 (2020); https://doi.org/10.1063/5.0021270 Submitted: 08 July 2020 . Accepted: 17 August 2020 . Published Online: 09 September 2020 Maria Soler, Alexis Scholtz, Rene Zeto, and Andrea M. Armani COLLECTIONS Paper published as part of the special topic on Coronavirus and Photonics This paper was selected as Featured This paper was selected as Scilight ARTICLES YOU MAY BE INTERESTED IN Short- and long-term photonics solutions for COVID-19 Scilight 2020, 371103 (2020); https://doi.org/10.1063/10.0001946 Deep learning of ultrafast pulses with a multimode fiber APL Photonics 5, 096106 (2020); https://doi.org/10.1063/5.0007037 Tunable microwave-photonic filtering with high out-of-band rejection in silicon APL Photonics 5, 096103 (2020); https://doi.org/10.1063/5.0015174 APL Photonics 5, 090901 (2020); https://doi.org/10.1063/5.0021270 5, 090901 © 2020 Author(s). APL Photonics PERSPECTIVE scitation.org/journal/app Engineering photonics solutions for COVID-19 Cite as: APL Photon. 5, 090901 (2020); doi: 10.1063/5.0021270 Submitted: 8 July 2020 • Accepted: 17 August 2020 • Published Online: 9 September 2020 Maria Soler,1 Alexis Scholtz,2 Rene Zeto,3 and Andrea M. Armani2,3,a) AFFILIATIONS 1 Nanobiosensors and Bioanalytical Applications Group (NanoB2A), Catalan Institute of Nanoscience and Nanotechnology (ICN2), CSIC, BIST and CIBER-BBN, Barcelona, Spain 2Department of Biomedical Engineering, University of Southern California, Los Angeles, California 90089, USA 3Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, Los Angeles, California 90089, USA Note: This paper is part of the APL Photonics Special Topic on Coronavirus and Photonics. -

Silicon Photonics Technologies
Silicon photonics technologies Highlights Handle more data—per watt, per fiber, per laser—for • Monolithic integration capabilities next-generation optical communications for cost-effective power, area and We are beyond the era where computers are simply connected. “Thinking” performance advantages machines, ranging from smart IoT sensors to AI-enabled hardware, are now a reality. • Electro-optic process design Transporting the massive volumes of data generated by these intelligent devices kits featuring a Cadence design environment with a comprehensive fuels bandwidth challenges. To meet this demand, there is a growing need for O-band library, path tool, models, semiconductor solutions that can leverage light to move more data. As a result, LVS and DRC optical communications chips are poised to enable new levels of performance • High-volume, advanced 300 mm in hyperscale data centers, cloud computing and 5G-driven network processing and controls that transformation. leverage CMOS infrastructure and methodologies The GLOBALFOUNDRIES (GF) silicon photonics (SiPh) foundry portfolio is designed to help you deliver more data faster, farther and more efficiently than • Automated high-volume inline test traditional CMOS technologies. The initial offering, 90WG, is an industry-first that brings CMOS-like processing and wafer acceptance criteria SiPh foundry solution. Built on a production-ready, differentiated 90 nm SOI controls to optics platform, the offering is manufactured at the GF 300 mm facility in East Fishkill, • World-class technology development NY, enabling you to leverage high-volume manufacturing along with advanced team delivering ongoing innovation, processing and controls that utilize proven CMOS manufacturing methodologies including laser attach and low-loss and infrastructure. -

Little Book of Photonics Careers
Little Book of Photonics Careers Contents What is photonics? .......................................... 3 What is a career in photonics? ................ 5 Photonics in lighting and screens .......... 7 Photonics in telecommunications .........8 Photonics in medicine and imaging .....9 Brian Murray ......................................................12 Yan Zhao ..............................................................14 Megan O’Brien .................................................16 John O`Dowd .................................................... 18 Colette McDonagh ...................................... 20 Frank Smyth .................................................22 Monika Zygowska ......................................24 Padraic Morrissey .....................................26 Andrea Pacheco ........................................28 Laura Horan ..................................................30 John Hayes ........................................................32 Agnieszka Gocalinska .................................34 Anil Jain ................................................................ 36 Una Buckley ..................................................... 38 Ludovic Caro .................................................... 40 Sinéad Byrne ....................................................42 Caroline Lai ........................................................44 1 The Irish Photonic Integration Centre (IPIC) funded by Science Foundation Ireland brings together over 150 researchers from five universities and institutes -

Photonics Professional Master's Flyer
PROFESSIONAL MASTER’S OPTION Earn Your Degree in Photonics Engineering A world-class program founded on state-of-the-art design and fabrication tools to en- gender deep understanding coupled to practical skills. The Department of Electrical, Computer and Energy Engineering at the University of Colorado Boulder is excited to offer the Photonics professional mas- Why Photonics Engineering? ter’s program covering high-demand talents such as computer-aided photonics design founded on a deep The 20th century was defined by the understanding of how light interacts with matter. growth of electronics, but the 21st century belongs to photonics. LEDs The program is offered under the College of Engi- neering and Applied Science’s Professional Mas- will light homes powered by solar pan- ter of Science or Master’s of Engineering degree. els. Laser 3D printing will transform Through core courses (offered on campus and manufacturing. New microscopes and through several online formats for maxium flexibility) telescopes will peer into the depths of and a broad slate of electives, students enrolled in the Photonics program may pursue a 9-credit hour living cells and distant galaxies. certificate or 30-credit hour degree. Photonics graduates will command Graduates will be poised to solve critical engineer- skills in design, fabrication and lab- ing challenges including monitoring our health and environment, producing and conserving our energy oratory practice to place them at the resources and creating the next generation of hu- forefront of these industries