Materials, Devices and Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
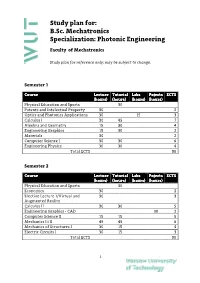
B.Sc. Mechatronics Specialization: Photonic Engineering
Study plan for: B.Sc. Mechatronics Specialization: Photonic Engineering Faculty of Mechatronics Study plan for reference only; may be subject to change. Semester 1 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Patents and Intelectual Property 30 2 Optics and Photonics Applications 30 15 3 Calculus I 30 45 7 Algebra and Geometry 15 30 4 Engineering Graphics 15 30 2 Materials 30 2 Computer Science I 30 30 6 Engineering Physics 30 30 4 Total ECTS 30 Semester 2 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Economics 30 2 Elective Lecture 1/Virtual and 30 3 Augmented Reality Calculus II 30 30 5 Engineering Graphics ‐ CAD 30 2 Computer Science II 15 15 5 Mechanics I i II 45 45 6 Mechanics of Structures I 30 15 4 Electric Circuits I 30 15 3 Total ECTS 30 1 Study Plan for B.Sc. Mechatronics (Spec. Photonic Engineering) Semester 3 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Elective Lecture 2/Introduction to 30 3 MEMS Calculus III 15 30 6 Mechanics of Structures II 15 15 4 Manufacturing Technology I 30 4 Fine Machine Design I 15 30 3 Electric Circuits II 30 3 Basics of Automation and Control I 30 15 4 Total ECTS 31 Semester 4 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Foreign Language 60 4 Elective Lecture 3/Photographic 30 3 techniques in image acqusition Elective Lecture 4 30 3 /Enterpreneurship Optomechatronics 30 30 5 Electronics I 15 15 2 Electronics II 15 1 Fine Machine Design II 15 15 3 Manufacturing Technology 30 2 Metrology 30 30 4 Total ECTS 27 Semester 5 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Marketing 30 2 Elective Lecture 5/ Electric 30 2 2 Study Plan for B.Sc. -

Chemical Engineering Education Graduate Education in Chemical Engineering
I • N • D • E • X GRADUATE EDUCATION ADVERTISEMENTS Akron, Uni versity of. .......... , .... ... .................. 321 Iowa State Uni versity .................. ... ....... ....... 360 Pensylvania State Uni versity ........................ 395 Alabama, University of ................................ 322 Johns Hopkins University .... .... .. .... .... .......... 361 Pittsburgh, University of .............................. 396 Alabama, Huntsville; Uni versity of.. .... .. ..... 323 Kansas, University of ............................... .... 362 Polytechnic University .. .... ... .... ........... .. ..... .. 397 Alberta, Uni versity of .. ........ .... .. .... ... ..... ..... .. 324 Kansas State University ............... ... ...... ........ 363 Princeton University ....................... .......... .. .. 398 Arizona, University of ....... .. .... .. .... ... .. ... ....... 325 Kentucky, Uni versity of ........................ .. ..... 364 Purdue University .. ........... ... ... ....... ... .... .... ... 399 Arizona State University ..... .. ... ...... ..... ......... 326 Lamar University .. ... ..... ..... ......... ........... .. ..... 430 Rensselaer Polytechnic Institute .... ...... .... ... .. 400 Auburn Uni versity .. ..... .. ... ..... .. .............. .... ... 327 Laval Universite ...................... ........... ...... .. .. 365 Rhode Island, University of.. .... ..... .. ... ..... .. ... 435 Bri gham Young Uni versity .............. ... .. ..... ... 427 Lehigh University .................................. .... ... 366 Rice University -

(12) United States Patent (10) Patent No.: US 8,298,686 B2 Uetani (45) Date of Patent: Oct
US008298686B2 (12) United States Patent (10) Patent No.: US 8,298,686 B2 Uetani (45) Date of Patent: Oct. 30, 2012 (54) COMPOSITION AND ORGANIC JP 2008-266459 A 11, 2008 PHOTOELECTRC CONVERTER USING THE WO 2008/O16091 A1 2/2008 SAME WO 2008/044585 A1 4, 2008 WO 2008/093822 A1 8/2008 (75) Inventor: Yasunori Uetani, Tsukuba (JP) WO 2008/0938.23 A1 8/2008 WO 2008/093831 A1 8/2008 (73) Assignee: Sumitomo Chemical Company, Limited, Tokyo (JP) OTHER PUBLICATIONS (*) Notice: Subject to any disclaimer, the term of this Youngkyoo Kim, et al., Organic Photovoltaic Devices Based on patent is extended or adjusted under 35 Blends of Regioregular Poly(3-hexylthiophene) and Poly(9.9- U.S.C. 154(b) by 124 days. dioctylfluorene-co-benzothiadiazole), Chemistry of Materials, vol. 16, No. 3, Nov. 1, 2004, pp. 4812-4818. (21) Appl. No.: 12/867,799 O. Inganäs, et al., "Low bandgap alternating polyfluorene copoly (22) PCT Filed: Feb. 17, 2009 mers in plastic photodiodes and Solar cells'. Applied Physics A: Materials Science & Processing, vol. 79, No. 1, Jun. 1, 2004, pp. (86). PCT No.: PCT/UP2009/053126 31-35. S371 (c)(1), Extended European Search Report issued Mar. 29, 2012 in European (2), (4) Date: Aug. 16, 2010 Patent Application No. 09713439.9. Christopher R. McNeill, et al., “Dual electron donor/electron accep (87) PCT Pub. No.: WO2009/104781 tor character of a conjugated polymer in efficient photovoltaic PCT Pub. Date: Aug. 27, 2009 diodes'. Applied Physics Letters, vol. 90, No. 19, May 9, 2007, pp. 193506-1-193506-3. -

Read About the Future of Packaging with Silicon Photonics
The future of packaging with silicon photonics By Deborah Patterson [Patterson Group]; Isabel De Sousa, Louis-Marie Achard [IBM Canada, Ltd.] t has been almost a decade Optics have traditionally been center design. Besides upgrading optical since the introduction of employed to transmit data over long cabling, links and other interconnections, I the iPhone, a device that so distances because light can carry the legacy data center, comprised of many successfully blended sleek hardware considerably more information off-the-shelf components, is in the process with an intuitive user interface that it content (bits) at faster speeds. Optical of a complete overhaul that is leading to effectively jump-started a global shift in transmission becomes more energy significant growth and change in how the way we now communicate, socialize, efficient as compared to electronic transmit, receive, and switching functions manage our lives and fundamentally alternatives when the transmission are handled, especially in terms of next- interact. Today, smartphones and countless length and bandwidth increase. As the generation Ethernet speeds. In addition, other devices allow us to capture, create need for higher data transfer speeds at as 5G ramps, high-speed interconnect and communicate enormous amounts of greater baud rate and lower power levels between data centers and small cells will content. The explosion in data, storage intensifies, the trend is for optics to also come into play. These roadmaps and information distribution is driving move closer to the die. Optoelectronic will fuel multi-fiber waveguide-to-chip extraordinary growth in internet traffic interconnect is now being designed interconnect solutions, laser development, and cloud services. -

Merging Photonics and Artificial Intelligence at the Nanoscale
Intelligent Nanophotonics: Merging Photonics and Artificial Intelligence at the Nanoscale Kan Yao1,2, Rohit Unni2 and Yuebing Zheng1,2,* 1Department of Mechanical Engineering, The University of Texas at Austin, Austin, Texas 78712, USA 2Texas Materials Institute, The University of Texas at Austin, Austin, Texas 78712, USA *Corresponding author: [email protected] Abstract: Nanophotonics has been an active research field over the past two decades, triggered by the rising interests in exploring new physics and technologies with light at the nanoscale. As the demands of performance and integration level keep increasing, the design and optimization of nanophotonic devices become computationally expensive and time-inefficient. Advanced computational methods and artificial intelligence, especially its subfield of machine learning, have led to revolutionary development in many applications, such as web searches, computer vision, and speech/image recognition. The complex models and algorithms help to exploit the enormous parameter space in a highly efficient way. In this review, we summarize the recent advances on the emerging field where nanophotonics and machine learning blend. We provide an overview of different computational methods, with the focus on deep learning, for the nanophotonic inverse design. The implementation of deep neural networks with photonic platforms is also discussed. This review aims at sketching an illustration of the nanophotonic design with machine learning and giving a perspective on the future tasks. Keywords: deep learning; (nano)photonic neural networks; inverse design; optimization. 1. Introduction Nanophotonics studies light and its interactions with matters at the nanoscale [1]. Over the past decades, it has received rapidly growing interest and become an active research field that involves both fundamental studies and numerous applications [2,3]. -

Experimental Photonics Multiple Post-Doctoral Positions Experimental Expertise in Any One of the Following Topics/Areas Is Highly Desired
Experimental Photonics Multiple Post-Doctoral Positions Experimental Expertise in any one of the following topics/areas is highly desired . Single photon level measurements , quantum communications . Computational imaging, super-resolution imaging, biomedical imaging . Quantum dots, 2D materials, quantum devices, quantum transport . Single molecule spectroscopy/imaging . Fluorescence microscopy . Optical manipulation of spin , ODMR, Magnetometry, NV centers . Nanofabication (Metasurfaces, plasmonics,silicon photonics) . Streak camera or time-correlated single photon counting experiments . Ultrafast spectroscopy, pump-probe measurements . Single nanoparticle/nanoantenna experiments . Coupling of single quantum emitters to nanophotonic structures . Cold atoms and quantum optics . Infrared spectroscopy, thermal emission measurements Please send your full CV and three representative publications to: [email protected] Prof. Zubin Jacob Birck Nanotechnology Center School of Electrical and Computer Engineering Purdue University, U.S.A. www.electrodynamics.org Zubin Jacob Research Group: Purdue University www.electrodynamics.org About the group Google Scholar Page: https://scholar.google.ca/citations?user=8FXvN_EAAAAJ&hl=en Main Research Areas: Casimir forces, quantum nanophotonics, plasmonics, metamaterials, Vacuum fluctuations, open quantum systems Weblink: www.electrodynamics.org Theory and Experiment Twitter: twitter.com/zjacob_group • Opportunity to closely interact with theorists and experimentalists within the group • Opportunity to travel -

Photonics Engineer
Photonics Engineer Antelope company Antelope DX develops a point-of-need diagnostic platform that allows consumers and healthcare professionals to have on-the-spot access to key health parameters. The Antelope technology aims to offer clinical lab performance with the ease-of-use of a pregnancy test at a consumer price tag. The platform is based on an innovative lab-on-chip technology that can perform a sensitive test on any bodily fluid, without requiring complex user operations or sample preparation. Role The Antelope Photonics Engineer is responsible for the design & development of the silicon photonic chip, located inside the Antelope consumable. He/she will also contribute largely to the optics and photonics aspects of associated hardware such as the Antelope reader. He/she will need to perform these product developments in a way that is compatible to IVD industry standards, including the generation of associated documentation. Responsibilities and duties • Photonics design & optimization of the sensing circuits. • Set up an optical/photonic system model to better predict and understand deviations from the norm by e.g. manufacturing tolerances. • Setting up characterisation, verification and QC equipment and methodologies for the photonic wafers & chips. • Support the design of the optical components of the read-out system. • Support the developmentt of the algorithmic framework that processes the optical signals to a diagnostic answer. • Support the development of R&D tools & methodologies from a system perspective to increase R&D efficiency, throughput and data generation. • Support the improvement of the R&D experimental setups, used to generate assay results. • Setting up testing and verification planning. -

Underwater Transformer
ALL ABOARD THE THE LAST A FORGOTTEN MICROGRIDS HYDROGEN TRAIN TRANSISTOR? REVOLUTION GO MOBILE Fuel cells go the Nanosheet devices and Why switching power A new and cleaner extra mile the end of Moore’s Law supplies triumphed way to power a ferry P. 06 P. 30 P. 36 P. 42 THIS UNTETHERED HALF HUMANOID WILL WORK ON OFFSHORE OIL RIGS AND GO DEEPER THAN FOR THE FOR THE TECHNOLOGY INSIDERTECHNOLOGY | 08.19 HUMAN DIVERS INSIDER P. 22 08.19 THE UNDERWATER TRANSFORMER Subscriber Address Box Zurich Instruments ∏ Arbitrary Waveform Generators ∏ Impedance Analyzers ∏ Lock-in Amplifiers ∏ Quantum Computing Control Systems experience LabOne LabOne® provides users with a platform independent instrument control including proven measurement methodo- logies. It's designed to offer a great amount of flexibility for instrument usage and assures quick and efficient ope- ration. The browser based user interface provides an outstanding toolset for time and frequency domain analysis as well as sophisticated support to set up control loops, making noise measure- ments or to interpret measurement data. Let's discuss your application Intl. +41 44 515 0410 USA 855-500-0056 (Toll Free) [email protected] www.zhinst.com CONTENTS_08.19 THE UNDER- WATER 30 THE LAST 36 THE QUIET 42 HAPPINESS IS 06 NEWS SILICON REMAKING OF A HYBRID-ELECTRIC 14 RESOURCES TRANSFORMER TRANSISTOR COMPUTER POWER FERRY 04 OPINION The next step in SUPPLIES The retrofitted 52 PAST FORWARD A startup founded by ex-NASA the evolution of the Here’s how the ship offers a clean, engineers wants to upend subsea transistor is the compact and efficient quiet ride for 4,500 nanosheet device. -

Illuminating the History and Expanding Photonics Education
Illuminating the History and Expanding Photonics Education An Interactive Qualifying Project submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE in partial fulfilment of the requirements for the degree of Bachelor of Science by Nicholas Marshall Brandon McLaughlin Date: 2nd June 2020 Report Submitted to: Worcester Polytechnic Institute Quinsigamond Community College Professor Douglas Petkie Worcester Polytechnic Institute This report represents work of WPI undergraduate students submitted to the faculty as evidence of a degree requirement. WPI routinely publishes these reports on its web site without editorial or peer review. For more information about the projects program at WPI, see http://www.wpi.edu/Academics/Projects. Abstract Photonics today is on the cusp of revolutionizing computing, just as it has already revolutionized communication, and becoming to this century what electricity was to the last (Sala, 2016). As the manifestation of mankind's millenia-spanning obsession with light, photonics evolved from optics, which itself developed over the long course of human history. That development has accelerated in the last several centuries, and today optics and photonics act as enablers for a variety of fields from biology to communication. Even so, most people don’t know just how essential optics and photonics are, and today those fields face a major staffing shortage. Most people don’t even know the basic principles of light’s behavior, with few formal education programs that focus on optics and photonics. In order to combat this, various initiatives have strived to drum up more interest in optics and photonics, with several focusing on pre-college age groups in order to get students involved sooner. -

Polyfluorene Has Emerged As an Attractive Material for Display
Highly efficient blue polyfluorene-based polymer light - emitting diodes through solvent vapor annealing T. Zhang, Y. Deng, S. Johnson and G. Liu* Environmental Energy Technologies Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720 Abstract A highly efficient blue polymer light emitting diode based on [poly(9,9-di(2-(2-(2- methoxy-ethoxy)ethoxy)ethyl)fluorenyl-2,7-diyl)] (PFOEO3) by solvent vapor annealing the polymer emitting layer is fabricated in a bi-layer device with the Al cathode. High electroluminescent efficiency is achieved by balancing charge injection. Better contact interface between polymer and cathode formed by making chain conformation reoriented with solvent vapor exposure. The effect of solvent vapor exposure on the potential barrier height of the interface is estimated using the Richardson-Schottky model. The maximum efficiency achieved 2.3 cd/A of the vapor exposed device from 0.9cd/A of the untreated pristine device. *Corresponding Author: Phone: (510) 486-7207; fax: (510) 486-7303; email: [email protected] 1 1.Introduction Conjugated polymer electroluminescence (EL) has been extensively studied for its applications in optoelectronic devices since the original report by Burroughes et al.[1] The solution based fabrication process for the polymer organic light emitting diode (OLED) has been recognized as a major advantage compared to the vacuum deposition process for small molecule OLED device. However, one major problem faced in the development of polymer light-emitting diodes is that of unbalanced carrier transport. One option is to use multi-layer devices with charge-injection or transporting layer. One limitation imposed by the solution process is layer mixing during the coating process for multilayered devices. -

Polyfluorene-Based Semiconductors Combined with Various Periodic
Progress in Polymer Science 37 (2012) 1192–1264 Contents lists available at SciVerse ScienceDirect Progress in Polymer Science j ournal homepage: www.elsevier.com/locate/ppolysci Polyfluorene-based semiconductors combined with various periodic table elements for organic electronics ∗ Ling-Hai Xie, Cheng-Rong Yin, Wen-Yong Lai, Qu-Li Fan, Wei Huang Key Laboratory for Organic Electronics & Information Displays (KLOEID) and Institute of Advanced Materials (IAM), Nanjing University of Posts & Telecommunications (NUPT), Nanjing 210046, China a r t i c l e i n f o a b s t r a c t Article history: Polyfluorenes have emerged as versatile semiconducting materials with applications in Received 12 April 2011 various polymer optoelectronic devices, such as light-emitting devices, lasers, solar cells, Received in revised form 8 February 2012 memories, field-effect transistors and sensors. Organic syntheses and polymerizations Accepted 10 February 2012 allow for the powerful introduction of various periodic table elements and their build- Available online 16 February 2012 ing blocks into -conjugated polymers to meet the requirements of organic devices. In this review, a soccer-team-like framework with 11 nodes is initially proposed to illus- Keywords: trate the structure–property relationships at three levels: chain structures, thin films -Conjugated polymers and devices. Second, the modelling of hydrocarbon polyfluorenes (CPFs) is summarized Band-gap engineering Light-emitting diodes within the framework of a four-element design principle, in which we have highlighted Photovoltaic cell polymorphic poly(9,9-dialkylfluorene)s with unique supramolecular interactions, various Field-effect transistors hydrocarbon-based monomers with different electronic structures, functional bulky groups Memories with steric hindrance effects and ladder-type, kinked, hyperbranched and dendritic confor- mations. -

Development of a First-Generation Miniature Multiple Reference Optical Coherence Tomography Imaging Device
Development of a first-generation miniature multiple reference optical coherence tomography imaging device Paul M. McNamara Roshan Dsouza Colm O’Riordan Seán Collins Peter O’Brien Carol Wilson Josh Hogan Martin J. Leahy Paul M. McNamara, Roshan Dsouza, Colm O’Riordan, Seán Collins, Peter O’Brien, Carol Wilson, Josh Hogan, Martin J. Leahy, “Development of a first-generation miniature multiple reference optical coherence tomography imaging device,” J. Biomed. Opt. 21(12), 126020 (2016), doi: 10.1117/1.JBO.21.12.126020. Downloaded From: http://biomedicaloptics.spiedigitallibrary.org/ on 01/02/2017 Terms of Use: http://spiedigitallibrary.org/ss/termsofuse.aspx Journal of Biomedical Optics 21(12), 126020 (December 2016) Development of a first-generation miniature multiple reference optical coherence tomography imaging device Paul M. McNamara,a,b,* Roshan Dsouza,a,b Colm O’Riordan,c Seán Collins,c Peter O’Brien,c Carol Wilson,b Josh Hogan,b and Martin J. Leahya aNational University of Ireland, School of Physics, Tissue Optics and Microcirculation Imaging Group, National Biophotonics and Imaging Platform, Galway H91 CF50, Ireland bCompact Imaging Inc., 897 Independence Avenue, Suite 5B, Mountain View, California 94043, United States cIrish Photonic Integration Centre (IPIC), Tyndall National Institute, Lee Maltings Complex, Dyke Parade, Cork T12 R5CP, Ireland Abstract. Multiple reference optical coherence tomography (MR-OCT) is a technology ideally suited to low-cost, compact OCT imaging. This modality is an extension of time-domain OCT with the addition of a partial mirror in front of the reference mirror. This enables extended, simultaneous depth scanning with the relatively short scan range of a miniature voice coil motor on which the scanning mirror is mounted.