Contrasting Size Evolution in Marine and Freshwater Diatoms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

World Ocean Thermocline Weakening and Isothermal Layer Warming
applied sciences Article World Ocean Thermocline Weakening and Isothermal Layer Warming Peter C. Chu * and Chenwu Fan Naval Ocean Analysis and Prediction Laboratory, Department of Oceanography, Naval Postgraduate School, Monterey, CA 93943, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-831-656-3688 Received: 30 September 2020; Accepted: 13 November 2020; Published: 19 November 2020 Abstract: This paper identifies world thermocline weakening and provides an improved estimate of upper ocean warming through replacement of the upper layer with the fixed depth range by the isothermal layer, because the upper ocean isothermal layer (as a whole) exchanges heat with the atmosphere and the deep layer. Thermocline gradient, heat flux across the air–ocean interface, and horizontal heat advection determine the heat stored in the isothermal layer. Among the three processes, the effect of the thermocline gradient clearly shows up when we use the isothermal layer heat content, but it is otherwise when we use the heat content with the fixed depth ranges such as 0–300 m, 0–400 m, 0–700 m, 0–750 m, and 0–2000 m. A strong thermocline gradient exhibits the downward heat transfer from the isothermal layer (non-polar regions), makes the isothermal layer thin, and causes less heat to be stored in it. On the other hand, a weak thermocline gradient makes the isothermal layer thick, and causes more heat to be stored in it. In addition, the uncertainty in estimating upper ocean heat content and warming trends using uncertain fixed depth ranges (0–300 m, 0–400 m, 0–700 m, 0–750 m, or 0–2000 m) will be eliminated by using the isothermal layer. -

Downloaded from the Coriolis Global Data Center in France (Ftp://Ftp.Ifremer.Fr)
remote sensing Article Impact of Enhanced Wave-Induced Mixing on the Ocean Upper Mixed Layer during Typhoon Nepartak in a Regional Model of the Northwest Pacific Ocean Chengcheng Yu 1 , Yongzeng Yang 2,3,4, Xunqiang Yin 2,3,4,*, Meng Sun 2,3,4 and Yongfang Shi 2,3,4 1 Ocean College, Zhejiang University, Zhoushan 316000, China; [email protected] 2 First Institute of Oceanography, Ministry of Natural Resources, Qingdao 266061, China; yangyz@fio.org.cn (Y.Y.); sunm@fio.org.cn (M.S.); shiyf@fio.org.cn (Y.S.) 3 Laboratory for Regional Oceanography and Numerical Modeling, Pilot National Laboratory for Marine Science and Technology, Qingdao 266071, China 4 Key Laboratory of Marine Science and Numerical Modeling (MASNUM), Ministry of Natural Resources, Qingdao 266061, China * Correspondence: yinxq@fio.org.cn Received: 30 July 2020; Accepted: 27 August 2020; Published: 30 August 2020 Abstract: To investigate the effect of wave-induced mixing on the upper ocean structure, especially under typhoon conditions, an ocean-wave coupled model is used in this study. Two physical processes, wave-induced turbulence mixing and wave transport flux residue, are introduced. We select tropical cyclone (TC) Nepartak in the Northwest Pacific ocean as a TC example. The results show that during the TC period, the wave-induced turbulence mixing effectively increases the cooling area and cooling amplitude of the sea surface temperature (SST). The wave transport flux residue plays a positive role in reproducing the distribution of the SST cooling area. From the intercomparisons among experiments, it is also found that the wave-induced turbulence mixing has an important effect on the formation of mixed layer depth (MLD). -

Influences of the Ocean Surface Mixed Layer and Thermohaline Stratification on Arctic Sea Ice in the Central Canada Basin J
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115, C10018, doi:10.1029/2009JC005660, 2010 Influences of the ocean surface mixed layer and thermohaline stratification on Arctic Sea ice in the central Canada Basin J. M. Toole,1 M.‐L. Timmermans,2 D. K. Perovich,3 R. A. Krishfield,1 A. Proshutinsky,1 and J. A. Richter‐Menge3 Received 22 July 2009; revised 19 April 2010; accepted 1 June 2010; published 8 October 2010. [1] Variations in the Arctic central Canada Basin mixed layer properties are documented based on a subset of nearly 6500 temperature and salinity profiles acquired by Ice‐ Tethered Profilers during the period summer 2004 to summer 2009 and analyzed in conjunction with sea ice observations from ice mass balance buoys and atmosphere‐ocean heat flux estimates. The July–August mean mixed layer depth based on the Ice‐Tethered Profiler data averaged 16 m (an overestimate due to the Ice‐Tethered Profiler sampling characteristics and present analysis procedures), while the average winter mixed layer depth was only 24 m, with individual observations rarely exceeding 40 m. Guidance interpreting the observations is provided by a 1‐D ocean mixed layer model. The analysis focuses attention on the very strong density stratification at the base of the mixed layer in the Canada Basin that greatly impedes surface layer deepening and thus limits the flux of deep ocean heat to the surface that could influence sea ice growth/ decay. The observations additionally suggest that efficient lateral mixed layer restratification processes are active in the Arctic, also impeding mixed layer deepening. Citation: Toole, J. -

Coastal Upwelling Revisited: Ekman, Bakun, and Improved 10.1029/2018JC014187 Upwelling Indices for the U.S
Journal of Geophysical Research: Oceans RESEARCH ARTICLE Coastal Upwelling Revisited: Ekman, Bakun, and Improved 10.1029/2018JC014187 Upwelling Indices for the U.S. West Coast Key Points: Michael G. Jacox1,2 , Christopher A. Edwards3 , Elliott L. Hazen1 , and Steven J. Bograd1 • New upwelling indices are presented – for the U.S. West Coast (31 47°N) to 1NOAA Southwest Fisheries Science Center, Monterey, CA, USA, 2NOAA Earth System Research Laboratory, Boulder, CO, address shortcomings in historical 3 indices USA, University of California, Santa Cruz, CA, USA • The Coastal Upwelling Transport Index (CUTI) estimates vertical volume transport (i.e., Abstract Coastal upwelling is responsible for thriving marine ecosystems and fisheries that are upwelling/downwelling) disproportionately productive relative to their surface area, particularly in the world’s major eastern • The Biologically Effective Upwelling ’ Transport Index (BEUTI) estimates boundary upwelling systems. Along oceanic eastern boundaries, equatorward wind stress and the Earth s vertical nitrate flux rotation combine to drive a near-surface layer of water offshore, a process called Ekman transport. Similarly, positive wind stress curl drives divergence in the surface Ekman layer and consequently upwelling from Supporting Information: below, a process known as Ekman suction. In both cases, displaced water is replaced by upwelling of relatively • Supporting Information S1 nutrient-rich water from below, which stimulates the growth of microscopic phytoplankton that form the base of the marine food web. Ekman theory is foundational and underlies the calculation of upwelling indices Correspondence to: such as the “Bakun Index” that are ubiquitous in eastern boundary upwelling system studies. While generally M. G. Jacox, fi [email protected] valuable rst-order descriptions, these indices and their underlying theory provide an incomplete picture of coastal upwelling. -
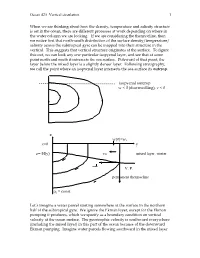
Ocean 423 Vertical Circulation 1 When We Are Thinking About How The
Ocean 423 Vertical circulation 1 When we are thinking about how the density, temperature and salinity structure is set in the ocean, there are different processes at work depending on where in the water column we are looking. If we are considering the thermocline, then we notice first that north-south distribution of the surface density/temperature/ salinity across the subtropical gyre can be mapped into their structure in the vertical. This suggests that vertical structure originates at the surface. To figure this out, we can look any one particular isopycnal layer, and see that at some point north and south it intersects the sea surface. Poleward of that point, the layer below the mixed layer is a slightly denser layer. Following stratigraphy, we call the point where an isopycnal layer intersects the sea-surface its outcrop. isopycnal outcrop w < 0 (downwelling), v < 0 z w(0)=we z=0 y z=-H(y) vG mixed layer, winter L.I. V. P. permanent thermocline = const Let’s imagine a water parcel starting somewhere at the surface in the northern half of the subtropical gyre. We ignore the Ekman layer, except for the Ekman pumping it produces, which we specify as a boundary condition on vertical velocity at the ocean surface. The geostrophic velocity is southward everywhere (including the mixed layer) in this part of the ocean because of the downward Ekman pumping. Imagine water parcels flowing southward in the mixed layer Ocean 423 Vertical circulation 2 (and no heating or cooling). A water parcel, when it encounters lighter water to the south will tend to follow its own isopycnal down out of the mixed layer. -

Implementation of an Ocean Mixed Layer Model in IFS
622 Implementation of an ocean mixed layer model in IFS Y. Takaya12, F. Vitart1, G. Balsamo1, M. Balmaseda1, M. Leutbecher1, F. Molteni1 Research Department 1European Centre for Medium-Range Weather Forecasts, Reading, UK 2Japan Meteorological Agency, Tokyo, Japan March 2010 Series: ECMWF Technical Memoranda A full list of ECMWF Publications can be found on our web site under: http://www.ecmwf.int/publications/ Contact: [email protected] c Copyright 2010 European Centre for Medium-Range Weather Forecasts Shinfield Park, Reading, RG2 9AX, England Literary and scientific copyrights belong to ECMWF and are reserved in all countries. This publication is not to be reprinted or translated in whole or in part without the written permission of the Director. Appropriate non-commercial use will normally be granted under the condition that reference is made to ECMWF. The information within this publication is given in good faith and considered to be true, but ECMWF accepts no liability for error, omission and for loss or damage arising from its use. Implementation of an ocean mixed layer model Abstract An ocean mixed layer model has been implemented in the ECMWF Integrated Forecasting System (IFS) in order to have a better representation of the atmosphere-upper ocean coupled processes in the ECMWF medium-range forecasts. The ocean mixed layer model uses a non-local K profile parameterization (KPP; Large et al. 1994). This model is a one-dimensional column model with high vertical resolution near the surface to simulate the diurnal thermal variations in the upper ocean. Its simplified dynamics makes it cheaper than a full ocean general circulation model and its implementation in IFS allows the atmosphere- ocean coupled model to run much more efficiently than using a coupler. -

Characterizing Transport Between the Surface Mixed Layer and the Ocean Interior with a Forward and Adjoint Global Ocean Transport Model
APRIL 2005 P R I M E A U 545 Characterizing Transport between the Surface Mixed Layer and the Ocean Interior with a Forward and Adjoint Global Ocean Transport Model FRANÇOIS PRIMEAU Department of Earth System Science, University of California, Irvine, Irvine, California (Manuscript received 1 July 2003, in final form 7 September 2004) ABSTRACT The theory of first-passage time distribution functions and its extension to last-passage time distribution functions are applied to the problem of tracking the movement of water masses to and from the surface mixed layer in a global ocean general circulation model. The first-passage time distribution function is used to determine in a probabilistic sense when and where a fluid element will make its first contact with the surface as a function of its position in the ocean interior. The last-passage time distribution is used to determine when and where a fluid element made its last contact with the surface. A computationally efficient method is presented for recursively computing the first few moments of the first- and last-passage time distributions by directly inverting the forward and adjoint transport operator. This approach allows integrated transport information to be obtained directly from the differential form of the transport operator without the need to perform lengthy multitracer time integration of the transport equations. The method, which relies on the stationarity of the transport operator, is applied to the time-averaged transport operator obtained from a three-dimensional global ocean simulation performed with an OGCM. With this approach, the author (i) computes surface maps showing the fraction of the total ocean volume per unit area that ventilates at each point on the surface of the ocean, (ii) partitions interior water masses based on their formation region at the surface, and (iii) computes the three-dimensional spatial distribution of the mean and standard deviation of the age distribution of water. -

Lecture 4: OCEANS (Outline)
LectureLecture 44 :: OCEANSOCEANS (Outline)(Outline) Basic Structures and Dynamics Ekman transport Geostrophic currents Surface Ocean Circulation Subtropicl gyre Boundary current Deep Ocean Circulation Thermohaline conveyor belt ESS200A Prof. Jin -Yi Yu BasicBasic OceanOcean StructuresStructures Warm up by sunlight! Upper Ocean (~100 m) Shallow, warm upper layer where light is abundant and where most marine life can be found. Deep Ocean Cold, dark, deep ocean where plenty supplies of nutrients and carbon exist. ESS200A No sunlight! Prof. Jin -Yi Yu BasicBasic OceanOcean CurrentCurrent SystemsSystems Upper Ocean surface circulation Deep Ocean deep ocean circulation ESS200A (from “Is The Temperature Rising?”) Prof. Jin -Yi Yu TheThe StateState ofof OceansOceans Temperature warm on the upper ocean, cold in the deeper ocean. Salinity variations determined by evaporation, precipitation, sea-ice formation and melt, and river runoff. Density small in the upper ocean, large in the deeper ocean. ESS200A Prof. Jin -Yi Yu PotentialPotential TemperatureTemperature Potential temperature is very close to temperature in the ocean. The average temperature of the world ocean is about 3.6°C. ESS200A (from Global Physical Climatology ) Prof. Jin -Yi Yu SalinitySalinity E < P Sea-ice formation and melting E > P Salinity is the mass of dissolved salts in a kilogram of seawater. Unit: ‰ (part per thousand; per mil). The average salinity of the world ocean is 34.7‰. Four major factors that affect salinity: evaporation, precipitation, inflow of river water, and sea-ice formation and melting. (from Global Physical Climatology ) ESS200A Prof. Jin -Yi Yu Low density due to absorption of solar energy near the surface. DensityDensity Seawater is almost incompressible, so the density of seawater is always very close to 1000 kg/m 3. -

NOAA Atlas NESDIS 81 WORLD OCEAN ATLAS 2018 Volume 1
NOAA Atlas NESDIS 81 WORLD OCEAN ATLAS 2018 Volume 1: Temperature Silver Spring, MD July 2019 U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Environmental Satellite, Data, and Information Service National Centers for Environmental Information NOAA National Centers for Environmental Information Additional copies of this publication, as well as information about NCEI data holdings and services, are available upon request directly from NCEI. NOAA/NESDIS National Centers for Environmental Information SSMC3, 4th floor 1315 East-West Highway Silver Spring, MD 20910-3282 Telephone: (301) 713-3277 E-mail: [email protected] WEB: http://www.nodc.noaa.gov/ For updates on the data, documentation, and additional information about the WOA18 please refer to: http://www.nodc.noaa.gov/OC5/indprod.html This document should be cited as: Locarnini, R.A., A.V. Mishonov, O.K. Baranova, T.P. Boyer, M.M. Zweng, H.E. Garcia, J.R. Reagan, D. Seidov, K.W. Weathers, C.R. Paver, and I.V. Smolyar (2019). World Ocean Atlas 2018, Volume 1: Temperature. A. Mishonov, Technical Editor. NOAA Atlas NESDIS 81, 52pp. This document is available on line at http://www.nodc.noaa.gov/OC5/indprod.html . NOAA Atlas NESDIS 81 WORLD OCEAN ATLAS 2018 Volume 1: Temperature Ricardo A. Locarnini, Alexey V. Mishonov, Olga K. Baranova, Timothy P. Boyer, Melissa M. Zweng, Hernan E. Garcia, James R. Reagan, Dan Seidov, Katharine W. Weathers, Christopher R. Paver, Igor V. Smolyar Technical Editor: Alexey Mishonov Ocean Climate Laboratory National Centers for Environmental Information Silver Spring, Maryland July 2019 U.S. DEPARTMENT OF COMMERCE Wilbur L. -

Oxygen, Apparent Oxygen Utilization, and Dissolved Oxygen Saturation
NOAA Atlas NESDIS 83 WORLD OCEAN ATLAS 2018 Volume 3: Dissolved Oxygen, Apparent Oxygen Utilization, and Dissolved Oxygen Saturation Silver Spring, MD July 2019 U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Environmental Satellite, Data, and Information Service National Centers for Environmental Information NOAA National Centers for Environmental Information Additional copies of this publication, as well as information about NCEI data holdings and services, are available upon request directly from NCEI. NOAA/NESDIS National Centers for Environmental Information SSMC3, 4th floor 1315 East-West Highway Silver Spring, MD 20910-3282 Telephone: (301) 713-3277 E-mail: [email protected] WEB: http://www.nodc.noaa.gov/ For updates on the data, documentation, and additional information about the WOA18 please refer to: http://www.nodc.noaa.gov/OC5/indprod.html This document should be cited as: Garcia H. E., K.W. Weathers, C.R. Paver, I. Smolyar, T.P. Boyer, R.A. Locarnini, M.M. Zweng, A.V. Mishonov, O.K. Baranova, D. Seidov, and J.R. Reagan (2019). World Ocean Atlas 2018, Volume 3: Dissolved Oxygen, Apparent Oxygen Utilization, and Dissolved Oxygen Saturation. A. Mishonov Technical Editor. NOAA Atlas NESDIS 83, 38pp. This document is available on-line at https://www.nodc.noaa.gov/OC5/woa18/pubwoa18.html NOAA Atlas NESDIS 83 WORLD OCEAN ATLAS 2018 Volume 3: Dissolved Oxygen, Apparent Oxygen Utilization, and Dissolved Oxygen Saturation Hernan E. Garcia, Katharine W. Weathers, Chris R. Paver, Igor Smolyar, Timothy P. Boyer, Ricardo A. Locarnini, Melissa M. Zweng, Alexey V. Mishonov, Olga K. Baranova, Dan Seidov, James R. -

Algae from the Arid Southwestern United States: an Annotated Bibliography
SERI/TR-231-1947 UC Category: 61a DE83011976 Algae from the Arid Southwestern United States: An Annotated Bibliography A Subcontract Report W. H. Thomas S. R. Gaines Institute of Marine Resources Scripps Institute of Oceanography University of California, San Diego La Jolla, California 92092 June 1983 Prepared under Subcontract No. XK-09111-1 SERI Technical Monitor: M. Lowenstein Solar Energy Research Institute A Division of Midwest Research Institute 1617 Cole Boulevard Golden, Colorado 80401 Prepared for the U.S. Department of Energy Contract No. EG-77-C-01-4042 Printed in the United States of America Available from: National Technical Information service U.S. Department of Commerce 5285 Port Royal Road Springfield, VA 22161 Price: Microfiche $4.50 Printed Copy $14.50 NOTICE This report was prepared as an account of work sponsored by the United States Government. Neither the United States nor the United States Department of Energy, nor any ·of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. FOREWORD This report is an annotated bibliography compiled by Scripps Institute of Oceanography for the Solar Energy Research Institute (SERI) under Subcontract XK-09111-1, using funds provided by the Biomass Energy Technology Divison of the Department of Energy. This report is provided to the research community in the hope that it will be an invaluable tool for future research in field collection and identification of microalgae in desert environments. -

Multispecies Fresh Water Algae Production for Fish Farming Using Rabbit Manure
fishes Article Multispecies Fresh Water Algae Production for Fish Farming Using Rabbit Manure Adandé Richard 1,*, Liady Mouhamadou Nourou Dine 1, Djidohokpin Gildas 1, Adjahouinou Dogbè Clément 1, Azon Mahuan Tobias Césaire 1, Micha Jean-Claude 2 and Fiogbe Didier Emile 1 1 Laboratory of Research on Wetlands (LRZH), Department of Zoology, Faculty of Sciences and Technology, University of Abomey-Calavi, B.P. 526 Cotonou, Benin; [email protected] (L.M.N.D.); [email protected] (D.G.); [email protected] (A.D.C.); [email protected] (A.M.T.C.); edfi[email protected] (F.D.E.) 2 Department of Biology, Research Unit in Environmental Biology, University of Namur, 5000 Namur, Belgium; [email protected] * Correspondence: [email protected]; Tel.: +229-95583595 Received: 27 July 2020; Accepted: 19 October 2020; Published: 30 November 2020 Abstract: The current study aims at determining the optimal usage conditions of rabbit manure in a multispecies fresh water algae production for fish farming. This purpose, the experimental design is made of six treatments in triplicate including one control T0,T1,T2,T3,T4,T5 corresponding respectively to 0, 300, 600, 900, 1200, 1500 g/m3 of dry rabbit manure put into buckets containing 40 L of demineralized water and then fertilized. The initial average seeding density is made of 4 103 2.5 102 cells/L of Chlorophyceae, 1.5 103 1 102 cells/L of Coscinodiscophyceae, × ± × × ± × 3 103 1.2 102 cells/L of Conjugatophyceae, 2.8 103 1.5 102 cells/L of Bascillariophyceae, × ± × × ± × and 2.5 103 1.4 102 cells/L of Euglenophyceae.