Anatomical and Physiological Similarities of Kidney in Different Experimental Animals Used for Basic Studies Harikesh Maurya*1, Tirath Kumar1 and Sunil Kumar2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Anatomy (Biology 2) Lecture Notes Updated July 2017 Instructor
Human Anatomy (Biology 2) Lecture Notes Updated July 2017 Instructor: Rebecca Bailey 1 Chapter 1 The Human Body: An Orientation • Terms - Anatomy: the study of body structure and relationships among structures - Physiology: the study of body function • Levels of Organization - Chemical level 1. atoms and molecules - Cells 1. the basic unit of all living things - Tissues 1. cells join together to perform a particular function - Organs 1. tissues join together to perform a particular function - Organ system 1. organs join together to perform a particular function - Organismal 1. the whole body • Organ Systems • Anatomical Position • Regional Names - Axial region 1. head 2. neck 3. trunk a. thorax b. abdomen c. pelvis d. perineum - Appendicular region 1. limbs • Directional Terms - Superior (above) vs. Inferior (below) - Anterior (toward the front) vs. Posterior (toward the back)(Dorsal vs. Ventral) - Medial (toward the midline) vs. Lateral (away from the midline) - Intermediate (between a more medial and a more lateral structure) - Proximal (closer to the point of origin) vs. Distal (farther from the point of origin) - Superficial (toward the surface) vs. Deep (away from the surface) • Planes and Sections divide the body or organ - Frontal or coronal 1. divides into anterior/posterior 2 - Sagittal 1. divides into right and left halves 2. includes midsagittal and parasagittal - Transverse or cross-sectional 1. divides into superior/inferior • Body Cavities - Dorsal 1. cranial cavity 2. vertebral cavity - Ventral 1. lined with serous membrane 2. viscera (organs) covered by serous membrane 3. thoracic cavity a. two pleural cavities contain the lungs b. pericardial cavity contains heart c. the cavities are defined by serous membrane d. -
Urinary System
URINARY SYSTEM Ján Líška DVM, PhD Institut of Histology and Embryology, Faculty of Medicine, Comenius University Urinary system • The kidneys are the organ with multiple functions: • filtration of the blood • excretion of metabolic waste products and related removal of toxins • maintenance blood volume • regulation of acid-base balance • regulation of fluid and electrolyte balance • production of the hormones The other components of urinary system are accessory. Their function is essentially in order to eliminate urine. Urinary system - anatomy • Kidney are located in the retroperitoneal space • The surface of the kidney is covered by a fibrous capsule of dense connective tissue. • This capsule is coated with adipose capsule. • Each kidney is attached to a ureter, which carries urine to the bladder and urine is discharged out through the urethra. ANATOMIC STRUCTURE OF THE KIDNEY RENAL LOBES CORTEX outer shell columns Excretory portion medullary rays MEDULLA medullary pyramids HILUM Collecting system blood vessels lymph vessels major calyces nerves RENAL PELVIS minor calyces ureter Cortex is the outer layer surrounding the internal medulla. The cortex contains renal corpuscles, convoluted parts of prox. and dist. tubules. Renal column: the renal tissue projection between two medullary pyramids which supports the cortex. Renal pyramids: the conical segments within the medulla. They contain the ductal apparatus and stright parts of the tubules. They posses papilla - having openings through which urine passes into the calyces. Each pyramid together with the associated overlying cortex forms a renal lobe. renal pyramid papilla minor calix minor calyx Medullary rays: are in the middle of cortical part of the renal lobe, consisting of a group of the straight portiones of nephrons and the collec- medullary rays ting tubules (only straight tubules). -

Urinary System
OUTLINE 27.1 General Structure and Functions of the Urinary System 818 27.2 Kidneys 820 27 27.2a Gross and Sectional Anatomy of the Kidney 820 27.2b Blood Supply to the Kidney 821 27.2c Nephrons 824 27.2d How Tubular Fluid Becomes Urine 828 27.2e Juxtaglomerular Apparatus 828 Urinary 27.2f Innervation of the Kidney 828 27.3 Urinary Tract 829 27.3a Ureters 829 27.3b Urinary Bladder 830 System 27.3c Urethra 833 27.4 Aging and the Urinary System 834 27.5 Development of the Urinary System 835 27.5a Kidney and Ureter Development 835 27.5b Urinary Bladder and Urethra Development 835 MODULE 13: URINARY SYSTEM mck78097_ch27_817-841.indd 817 2/25/11 2:24 PM 818 Chapter Twenty-Seven Urinary System n the course of carrying out their specific functions, the cells Besides removing waste products from the bloodstream, the uri- I of all body systems produce waste products, and these waste nary system performs many other functions, including the following: products end up in the bloodstream. In this case, the bloodstream is ■ Storage of urine. Urine is produced continuously, but analogous to a river that supplies drinking water to a nearby town. it would be quite inconvenient if we were constantly The river water may become polluted with sediment, animal waste, excreting urine. The urinary bladder is an expandable, and motorboat fuel—but the town has a water treatment plant that muscular sac that can store as much as 1 liter of urine. removes these waste products and makes the water safe to drink. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Anatomy of the Pig
Hands on Workshop: Lecture for Animal Workshop Anatomy of the pig Jong Man Kim1, Hae Won Lee2 Sungkyunkwan University1, Seoul National University2, Korea Introduction The digestive system of swine has anatomic differences from humans. However, the physiology of digestion remains similar to humans. In spite of the anatomic differences, the pig has been used extensively as a gastro- intestinal model. Most of the classical models involving the digestive system have been related to nutritional stud- ies to study digestion of the pig and for studying human digestive phenomenon. More recently endoscopic and laparoscopic surgical models have been developed and used extensively in the swine. The size and function of structures such as the biliary system and pancreatic duct make them amenable for studying human sized equip- ment and biomaterial implants. Surgical modifications have made the intestinal tract amenable to the study of surgical and chronic fistulation procedures. Laparoscopic surgery has replaced many open operations. The procedures described are commonly performed laparoscopically by many general surgeons but require practice. The porcine model is ideal to train surgeons in laparoscopic procedures since porcine anatomy is generally similar to humans with some minor differences. Liver 1. Morphological feature In the human, the liver morphologically consists of 4 lobes; the left, right, quadrate, and caudate lobes al- though the functional anatomy is more important than the morphological one, which is rarely used in clinical field. Unlikely to the human, the porcine liver consists of 5 lobes; the left lateral and medial, right lateral and medial, and caudate lobes. In the ventral view, 4 lobes are seen; the left lateral, left medial, right medial, and right lateral lobes in sequence from left to right. -
Ultra-Structure of Kidney the Excretory System Is Responsible for Filtering Wastes from the Blood and Both Forming and Secreting Urine
Ultra-Structure of kidney The excretory system is responsible for filtering wastes from the blood and both forming and secreting urine. These functions help to maintain the composition and volume of body fluids. Although it has far-reaching effects, the urinary system is relatively simple anatomically and consists of: Kidneys, Ureters, Bladder, and Urethra. The main organs are the kidneys, which filter blood and produce urine. The other parts are simply accessory structures for the transport and storage of urine. During the normal breakdown of protein and nucleic acids, nitrogen is released into the bloodstream. Some of this nitrogen is recycled to make new cellular products, but most of it is disposed of. The body has to have a way to rid itself of this unused nitrogen, as high levels in the blood can be toxic. Most of the nitrogen is bound with hydrogen as NH3 (ammonia), which is readily dissolved in water. For this reason, fish are able to excrete much of their nitrogen by simple diffusion into the surrounding water. Terrestrial animals have a different way of ridding their bodies of excess nitrogen. It is either excreted as uric acid or urea. Animals that are concerned about water loss, such as birds and reptiles, excrete the more concentrated uric acid as a pasty white material. Mammals, on the other hand, can excrete urea, along with more water. The mixture of urea, water, and other wastes is called 'urine.' Urine is still very concentrated in comparison to the blood, and the system that facilitates this concentration is the 'urinary system. -
Urinary System
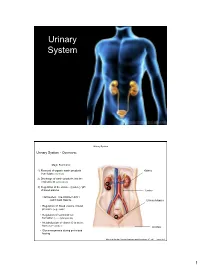
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels -
The Urinary System
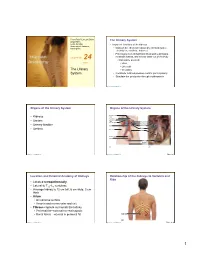
PowerPoint® Lecture Slides The Urinary System prepared by Leslie Hendon • Important functions of the kidneys University of Alabama, Birmingham • Maintain the chemical consistency of blood (water, electrolytes, acid/base balance) • Filter many liters of fluid from blood and send toxins, metabolic wastes, and excess water out of the body C H A P T E R 24 • Main waste products Part 1 • Urea • Uric acid The Urinary • Creatinine System • Contribute to blood pressure control (renin system) • Stimulate rbc production through erythropoietin Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Organs of the Urinary System Organs of the Urinary System Hepatic veins • Kidneys (cut) Esophagus (cut) Ureters Inferior vena • cava Renal artery Adrenal gland Renal hilum • Urinary bladder Aorta Renal vein Kidney • Urethra Iliac crest Ureter Rectum (cut) Uterus Urinary bladder Urethra (a) (b) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 24.1 Location and External Anatomy of Kidneys Relationship of the Kidneys to Vertebra and Ribs • Located retroperitoneally • Lateral to T12–L3 vertebrae • Average kidney is 12 cm tall, 6 cm wide, 3 cm thick • Hilum • On concave surface • Vessels and nerves enter and exit • Fibrous capsule surrounds the kidney • Perirenal fat—external to renal capsule • Renal fascia—external to perirenal fat 12th rib (b) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 24.2b 1 Position of the Kidneys with in the Posterior Internal -

(A) Adrenal Gland Inferior Vena Cava Iliac Crest Ureter Urinary Bladder
Hepatic veins (cut) Inferior vena cava Adrenal gland Renal artery Renal hilum Aorta Renal vein Kidney Iliac crest Ureter Rectum (cut) Uterus (part of female Urinary reproductive bladder system) Urethra (a) © 2018 Pearson Education, Inc. 1 12th rib (b) © 2018 Pearson Education, Inc. 2 Renal cortex Renal column Major calyx Minor calyx Renal pyramid (a) © 2018 Pearson Education, Inc. 3 Cortical radiate vein Cortical radiate artery Renal cortex Arcuate vein Arcuate artery Renal column Interlobar vein Interlobar artery Segmental arteries Renal vein Renal artery Minor calyx Renal pelvis Major calyx Renal Ureter pyramid Fibrous capsule (b) © 2018 Pearson Education, Inc. 4 Cortical nephron Fibrous capsule Renal cortex Collecting duct Renal medulla Renal Proximal Renal pelvis cortex convoluted tubule Glomerulus Juxtamedullary Ureter Distal convoluted tubule nephron Nephron loop Renal medulla (a) © 2018 Pearson Education, Inc. 5 Proximal convoluted Peritubular tubule (PCT) Glomerular capillaries capillaries Distal convoluted tubule Glomerular (DCT) (Bowman’s) capsule Efferent arteriole Afferent arteriole Cells of the juxtaglomerular apparatus Cortical radiate artery Arcuate artery Arcuate vein Cortical radiate vein Collecting duct Nephron loop (b) © 2018 Pearson Education, Inc. 6 Glomerular PCT capsular space Glomerular capillary covered by podocytes Efferent arteriole Afferent arteriole (c) © 2018 Pearson Education, Inc. 7 Filtration slits Podocyte cell body Foot processes (d) © 2018 Pearson Education, Inc. 8 Afferent arteriole Glomerular capillaries Efferent Cortical arteriole radiate artery Glomerular 1 capsule Three major renal processes: Rest of renal tubule 11 Glomerular filtration: Water and solutes containing smaller than proteins are forced through the filtrate capillary walls and pores of the glomerular capsule into the renal tubule. Peritubular 2 capillary 2 Tubular reabsorption: Water, glucose, amino acids, and needed ions are 3 transported out of the filtrate into the tubule cells and then enter the capillary blood. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
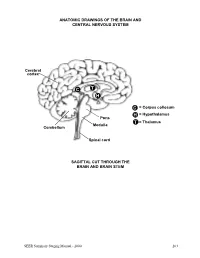
Brain and Central Nervous System
ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM Cerebral cortex C T H C = Corpus collosum H = Hypothalamus Pons T = Thalamus Medulla Cerebellum Spinal cord SAGITTAL CUT THROUGH THE BRAIN AND BRAIN STEM SEER Summary Staging Manual - 2000 263 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM 2 1 3 4 7 5 8 6 SAGITTAL CUT THROUGH THE HUMAN HEAD WITH CEREBRUM IN PLACE The cerebrum is comprised of the: 1 Frontal lobe 2 Parietal lobe 3 Temporal lobe 4 Occipital lobe Other parts of the brain include: 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) 264 SEER Summary Staging Manual - 2000 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM A B C D E 7 5 6 8 F SAGITTAL CUT THROUGH THE HUMAN HEAD Internal anatomy of the brain: A Inner surface of right hemisphere of cerebrum B Corpus callosum C Velum interpositum D Middle commissure E Third ventricle F Fourth ventricle Other parts of the brain (as on previous drawing): 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) SEER Summary Staging Manual - 2000 265 BRAIN AND CEREBRAL MENINGES C70.0, C71.0-C71.9 Supratentorial (S) or Infratentorial (I) C70.0 Cerebral meninges C71.0 Cerebrum ? (S) C71.1 Frontal lobe (S) C71.2 Temporal lobe (S) C71.3 Parietal lobe (S) C71.4 Occipital lobe (S) C71.5 Ventricle, NOS (S) C71.6 Cerebellum, NOS (I) C71.7 Brain stem (I) C71.8 Overlapping lesion of brain ? C71.9 Brain, NOS ? ?See Note 1. SUMMARY STAGE 1 Localized only Supratentorial tumor confined to: Cerebral hemisphere (cerebrum) or meninges of cerebral hemisphere -

Renal Ultrasound (Basic Principles) BMUS Study Day
Renal Ultrasound (Basic Principles) BMUS Study Day Rosie Conlon Clinical Specialist Sonographer NATIONAL REHABILITATION HOSPITAL Dun laoghaire, Dublin Sat 15th October 2016 WHY? “Bones can break, muscles can atrophy, glands can loaf about and even the brain can sleep without immediate danger to survival. BUT when the kidneys fail…. Neither bone, muscle gland nor brain could carry on.” Homer William Smith, “The Evolution of the Kidney”, Lectures on the Kidney (1943). Renal Ultrasound (Basic Principles) and BMUS Study Case PREPARATION • 500mls 1 hour before, avoiding • Operator Dependant micturition. • Catheter clamped 1.5 hours • Real Time before. • Fluids by PEG 1.5 hours before. • Reproducible • Non-invasive • Inspiration WHAT? - PROTOCOL • Both Kidneys • Urinary Bladder • +/- Residual Volume • Pelvic Surveillance • Aorta • Local protocol pertinent to the population • Full bladder The most important step in diagnosis is realising that it might exist. Renal Ultrasound (Basic Principles) and BMUS Study Case RIGHT KIDNEY-TECHNIQUE • A 3.5-5 MHz probe is typically used to scan the kidney. For the right kidney, have the patient lie supine and place the probe in the right lower intercostal space in the midaxillary line. Use the liver as your “acoustic window” and aim the probe slightly posteriorly (toward the kidney). Gently rock the probe (up and down or side to side) to scan the entire kidney. If needed, you can have the patient inspire or exhale, which allows for subtle movement of the kidney. • Obtain longitudinal (long axis) and transverse (short axis) views. Renal Ultrasound (Basic Principles) and BMUS Study Case ANATOMY Hepatic Veins Spleen Celiac axis Liver SMA Left Right Renal artery kidney kidney Renal vein Renal Ultrasound (Basic Principles) and BMUS Study Case NORMAL Renal Ultrasound (Basic Principles) and BMUS Study Case LEFT KIDNEY-TECHNIQUE • For the left kidney have the patient lie supine or in the right lateral decubitus position.