Product Allergy and Ingredient List (P.A.I.L.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Data Sheet TSP - TRISODIUM PHOSPHATE
Safety Data Sheet TSP - TRISODIUM PHOSPHATE Date of Revision: 2/11/2015 Section 1 – Chemical Product and Company Identification Product/Chemical Name: Trisodium Phosphate dodecahydrate Chemical Formula: Na3PO4*12H2O CAS Number: 10101-89-0 Other Designations: TSP; trisodium orthophosphate; tribasic; tertiary sodium phosphate; trisodium phosphate Derivation: Prepared by combining proper proportions of phosphoric acid and soda to form disodium phosphate, then adding a caustic soda Supplied by: PRO Chemical & Dye 126 Shove Street Fall River, MA 02724 Emergency Telephone Numbers: 800-255-3924 ChemTel. (United States) + 1 01 813-248-0585 (Outside the United States) 1. Section 2 - Hazards Identification HMIS ***** Emergency Overview ***** H 3 MAY CAUSE EYE INJURY. CAUSES SKIN IRRITATlON. MAYBE HARMFUL IF SWALLOWED. F 0 Potential Health Effects R 0 PPE Primary Entry Routes: Inhalation, ingestion or skin contact. Sec. 8 Target Organs: Skin, digestive tract. HAZARDS IDENTIFICATION Classification of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA DCS) Skin corrosion (Category I B). H314 Serious eye damage (Category I). H318 GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. Precautionary Statement(s) P260 Do not breathe dust or mist. P264 Wash skin thoroughly after handling. P280 Wear protective gloves protective clothing/ eye protection/ face protection. P301 + P330 + P331 IF SWALLOWED: rinse mouth. DO NOT induce vomiting. P303 + P36l + P353 IF ON SKIN (or hair): Remove. Take off immediately all contaminated clothing. Rinse skin with water / shower. P304 + P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. -

CO2 Capture from Flue Gas Using Amino Acid Salt Solutions
CO2 capture from flue gas using amino acid salt solutions Jacco van Holst, Patricia. P. Politiek, John P. M. Niederer, Geert F. Versteeg* University of Twente, Faculty Science and Technology (UT TNW), P.O. Box 217, 7500 AE Enschede, The Netherlands Abstract An initial kinetic study was performed on the reaction of CO2 with various potassium amino acid salt solutions at 298 K. Kinetics were measured at 0.5 kmol/m3, reason for which only apparent ki- netic constants are presented. The results were compared with the work of Kumar et al. [1] and Penny and Ritter [2]. Keywords: CO2, flue gas treatment, kinetics, potassium amino acid salt solutions Introduction One of the most alarming global environmental problems of today is the increase of the natural greenhouse effect. This problem is mainly caused by the increasing atmospheric carbon dioxide concentration due to the burning of fossil fuels for power generation. To reduce these problems, the carbon dioxide emissions from flue and fuel gases produced in combustion and gasification proc- esses in power plants have to be decreased by efficiency improvements and carbon dioxide capture. The removal of acid gases such as carbon dioxide, H2S or COS by absorption in aqueous alkanola- mine solutions is widely used in the chemical industry. Carbon dioxide reacts with primary and sec- ondary amines, reaching an equilibrium of carbamate, bicarbonate, and carbonate species. The ini- tial absorption reaction is the formation of the carbamate, which can then undergo hydrolysis to the bicarbonate and, if conditions such as pH are suitable, the carbonate species. The degree of hydroly- sis of the carbamate is determined by parameters such as amine concentration, solution pH, and the chemical stability of the carbamate [3]. -

Estimation of Phosphorus and Nitrogen Waste in Rainbow Trout (Oncorhynchus Mykiss, Walbaum, 1792) Diets Including Different Inorganic Phosphorus Sources
animals Article Estimation of Phosphorus and Nitrogen Waste in Rainbow Trout (Oncorhynchus mykiss, Walbaum, 1792) Diets Including Different Inorganic Phosphorus Sources Maria Consolación Milián-Sorribes 1, Ana Tomás-Vidal 1, David S. Peñaranda 1 , Laura Carpintero 2, Juan S. Mesa 2, Javier Dupuy 2, Andrés Donadeu 2, Judit Macías-Vidal 2 and Silvia Martínez-Llorens 1,* 1 Aquaculture and Biodiversity Research Group, Institute of Animal Science and Technology, Universitat Politècnica de València, Camino de Vera 14, 46071 València, Spain; [email protected] (M.C.M.-S.); [email protected] (A.T.-V.); [email protected] (D.S.P.) 2 R&D Department, Global Feed, S.L.U., Tervalis Group, Av. Francisco Montenegro s/n, 21001 Huelva, Spain; [email protected] (L.C.); [email protected] (J.S.M.); [email protected] (J.D.); [email protected] (A.D.); [email protected] (J.M.-V.) * Correspondence: [email protected] Simple Summary: Aquaculture effluents with high levels of phosphorus (P) and nitrogen (N) contribute to eutrophication in the aquatic ecosystem. The environmental impact of phosphorus and N aquaculture waste may be diminished by modifying diet ingredients that improve phosphorous (P) digestibility, and therefore, reduce the P in metabolic waste. The content of P in fishmeal is high Citation: Milián-Sorribes, M.C.; Tomás-Vidal, A.; Peñaranda, D.S.; (30 g/kg), but the inclusion of fishmeal in the diet is reducing due to its high costs and limited Carpintero, L.; Mesa, J.S.; Dupuy, J.; accessibility; therefore, the addition of an inorganic P source is necessary to ensure a satisfactory Donadeu, A.; Macías-Vidal, J.; level of available P in fish diets. -

SALTS of FATTY ACIDS
SALTS of FATTY ACIDS Prepared at the 33rd JECFA (1988), published in FNP 38 (1988) and in FNP 52 (1992). Metals and arsenic specifications revised at the 55th JECFA (2000). An ADI 'not specified' was established at the 33rd JECFA (1988) SYNONYMS INS No. 470 DEFINITION These products consist of calcium, potassium or sodium salts of commercial myristic, oleic, palmitic, stearic, acids or mixtures of these acids from edible fats and oils. The article of commerce can be further specified by: - saponification value, - solidification point for the fatty acids obtained from the salts, - iodine value, - residue on ignition including assay of the cation, and - moisture content Assay Not less than 95% total fatty acid salts, dry weight basis DESCRIPTION Hard, white or faintly yellowish, somewhat glossy and crystalline solids or semi-solids or white or yellowish-white powder FUNCTIONAL USES Anticaking agent, emulsifier CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Potassium and sodium salts are soluble in water and ethanol; calcium salts are insoluble in water, ethanol and ether Test for cations Heat 1 g of the sample with a mixture of 25 ml of water and 5 ml of hydrochloric acid. Fatty acids are liberated, floating as a solid or oil layer on the surface which is soluble in hexane. After cooling, aqueous layer is decanted and evaporated to dryness. Dissolve the residue in water and test for the appropriate cation. Fatty acid composition Using the Method of Assay, identify the individual fatty sample. The fatty acid(s) in primary abundance should conform to those declared on the label of the product PURITY Free fatty acids Not more than 3% Measure free fatty acids as directed in the method Free Fatty Acids. -

Brochure-Product-Range.Pdf
PRODUCT RANGE 2015 edition ANSI Standard 60 NSF® CERTIFIED HALAL M ISLAMIC FOOD AND NUTRITION ® COUNCIL OF AMERICA Rue Joseph Wauters, 144 ISO 9001:2008 (Quality) / OHSAS 18001:2007 (Health/ B-4480 Engis Safety) / ISO 14001:2004 (Environment) / ISO 22000:2005 www.globulebleu.com (Food Safety) / FSSC 22000:2013 (Food Safety). Tel. +32 (0) 4 273 93 58 Our food grade phosphates are allergen free, GMO free, Fax. +32 (0) 4 275 68 36 BSE/TSE free. www.prayon.com mail. [email protected] Design by www.prayon.com PRODUCT RANGE | 11 TABLE OF CONTENTS HORTICULTURE APPLICATIONS HORTIPRAY® RANGE FOR HORTICULTURE* FOOD AND INDUSTRIAL APPLICATIONS PRODUCT NAME Bulk density P O pH N-NH Made 2 5 4 MONOAMMONIUM PHOSPHATE - NH4H2PO4 in 3 3 % 1% % Sodium orthophosphates ................................................................................... 03 g/cm lbs/ft indicative indicative indicative Water-soluble fertilisers. Sodium pyrophosphates .................................................................................... 04 HORTIPRAY® MAP Horticultural Grade 0.9 56 61 4.5 12 Sodium tripolyphosphates ................................................................................. 05 HORTIPRAY® MAP 12.60 Horticultural Grade 0.9 56 60 5 12.1 Water-soluble fertilisers; Sodium polyphosphates ..................................................................................... 06 HORTIPRAY® MAP anticalc Horticultural Grade 0.9 56 61 4.5 12 preventive action against clogging. Potassium orthophosphates ............................................................................. -

Spray-Dried Monocalcium Phosphate Monohydrate for Soluble Phosphate Fertilizer Khouloud Nasri, Hafed El Feki, Patrick Sharrock, Marina Fiallo, Ange Nzihou
Spray-Dried Monocalcium Phosphate Monohydrate for Soluble Phosphate Fertilizer Khouloud Nasri, Hafed El Feki, Patrick Sharrock, Marina Fiallo, Ange Nzihou To cite this version: Khouloud Nasri, Hafed El Feki, Patrick Sharrock, Marina Fiallo, Ange Nzihou. Spray-Dried Monocal- cium Phosphate Monohydrate for Soluble Phosphate Fertilizer. Industrial and engineering chemistry research, American Chemical Society, 2015, 54 (33), p. 8043-8047. 10.1021/acs.iecr.5b02100. hal- 01609207 HAL Id: hal-01609207 https://hal.archives-ouvertes.fr/hal-01609207 Submitted on 15 Jan 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Spray-Dried Monocalcium Phosphate Monohydrate for Soluble Phosphate Fertilizer Khouloud Nasri and Hafed El Feki Laboratory of Materials and Environmental Sciences, Faculty of Sciences of Sfax, Soukra Road km 4B. P. no 802−3038, Sfax, Tunisia Patrick Sharrock* and Marina Fiallo Université de Toulouse, SIMAD, IUT Paul Sabatier, Avenue Georges Pompidou, 81104 Castres, France Ange Nzihou Centre RAPSODEE, Université de Toulouse, Mines Albi, CNRS, Albi, France ABSTRACT: Monocalcium phosphate monohydrate (MCPM) was obtained by water extraction of triple superphosphate. The solubility of MCPM is 783.1 g/L, and is entirely soluble. Saturated MCPM solution dissociates into free phosphoric acid and monetite (CaHPO4), but evaporation to dryness by spray drying forms MCPM during crystallization. -

DP70460.Pdf (4.893Mb)
HEAT CHAHGES ACCGKPABYIIJG ABSGRFTIOH EQUILIBRIA IB SOLTJTIQH. D. C. UCETSBVAUfER C h e w . LD Sill ,/V\7 od L VVC\ I If) <2 D-C. C o ' :o Thesim submitted to the Faculty of the Graduate School of the TJnivsrsity of Maryland in partial fulfillment of the requirements for the degree of Doctor of Phil osophy* A 1 9 fc 6 .. UMI Number: DP70460 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI DP70460 Published by ProQuest LLC (2015). Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKHQWLXDGMEHT The writer wishes to express hie appreciation and thaiifcs to Dr* Hell S. Gordon, Head of the Depart ment of Chemistry of the University of Maryland who dlreetcd this work and gate helpful suggestions and advice* TABLE OF G CH U M S X* Introduct 1 q h -—-*——— «*►»»««*«*»*«»»*— «■»»*-«««»»» x . Historical He view-----— ----- —■—— — — Z Heat of Absorption of Liquids art Gases- 1 4 Heat of Coagulation --- -- ™ -i^ * General Methods and Materials--— — — — — 2H of G els— 38H ----- - --- — ;S6 ------ Froeebnre——— ——-——32 If# Experiiaenial Hes’nlts-— --— — — — — ---- g*> T. Hubimavy C oixdua i OHS— —— — -— —— 61 FX* Lit eratare Gi ted :——— Introduction Adscript ion has for a long time teen a well recognised phenomenon and numerous investigations have been mad® on the nature and magnitude of the energy changes involved* Some of the measurementa on the adsorption of gases have shown ti&se changes to be enormous. -

Vaccine Excipient Table
Vaccine Excipient Summary Excipients Included in U.S. Vaccines, by Vaccine In addition to weakened or killed disease antigens (viruses or bacteria), vaccines contain very small amounts of other ingredients – excipients. Some excipients are added to a vaccine for a specific purpose. These include: Preservatives, to prevent contamination. For example, thimerosal. Adjuvants, to help stimulate a stronger immune response. For example, aluminum salts. Stabilizers, to keep the vaccine potent during transportation and storage. For example, sugars or gelatin. Others are residual trace amounts of materials that were used during the manufacturing process and removed. These can include: Cell culture materials, used to grow the vaccine antigens. For example, egg protein, various culture media. Inactivating ingredients, used to kill viruses or inactivate toxins. For example, formaldehyde. Antibiotics, used to prevent contamination by bacteria. For example, neomycin. The following table lists substances, other than active ingredients (i.e., antigens), shown in the manufacturers’ package insert (PI) as being contained in the final formulation of each vaccine. Note: Substances used in the manufacture of a vaccine but not listed as contained in the final product (e.g., culture media) can be found in each PI, but are not shown on this table. Each PI, which can be found on the FDA’s website (see below) contains a description of that vaccine’s manufacturing process, including the amount and purpose of each substance. In most PIs, this information is found -

General Properties of the Alkaline Phosphates: - Major Food and Technical Applications
Phosphorus Research Bulletin Vol. 15 (2004) p. 85-94 General Properties of the Alkaline Phosphates: - Major Food and Technical Applications P.HOURANT Deputy Business Line Manager, Prayon S.A., Business Unit Phosphates, Rue Joseph Wauters, 144 4480 Engis, Belgium; E-mail: [email protected] INTRODUCTION The alkaline phosphates are used for many food and technical applications. Phosphates have two characteristics that explain their four main properties: buffer agent, sequestering power, dispersing power and water holding capability. Those properties allow phosphates to be used in many food and technical applications. The main food applications are meat and seafood processing, baking and processed cheese, but others such as cereals, French fries, fruits and vegetables, beverages, noodles and so on also may need the use of phosphates. On the technical side, the main applications are the detergent products, the water treatment and the metal treatment. As for the food, many other applications require phosphates such as ceramics, bone china, paper and paints,... In meat products, phosphates salts interact in a unique way to bind water with proteins and improve the tenderness in meats. Treated products will maintain their juicy appearance as well as their natural nutritional properties texture and colour. In fish and seafood products, phosphates salts allow the retention of the natural juices of frozen fish fillets, prawns, shrimps, scallops and other seafood. Phosphates also help prevent the build-up of struvite crystals in tinned tuna and crabmeat. In processed cheese, phosphates are crucially important in the production of processed cheese. These products ensure a homogeneous and uniform melt of raw cheese and product stability. -
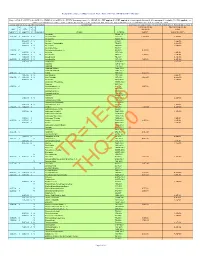
Composite Worker Air RSL May 2021 HQ10
Regional Screening Level (RSL) Composite Worker Ambient Air Table (TR=1E-06, HQ=1) May 2021 Key: I = IRIS; P = PPRTV; O = OPP; A = ATSDR; C = Cal EPA; X = PPRTV Screening Level; H = HEAST; W = TEF applied; E = RPF applied; G = user's guide Section 5; M = mutagen; V = volatile; R = RBA applied ; c = cancer; n = noncancer; * = where: n SL < 100X c SL; ** = where n SL < 10X c SL; SSL values are based on DAF=1; m = ceiling limit exceeded; s = Csat exceeded. Toxicity and Chemical-specific Information Contaminant Carcinogenic Target Risk (TR) = 1E-06 Noncancer Hazard Index (HI) = 1 k k v Carcinogenic SL Noncarcinogenic SL IUR e RfCi e o TR=1E-06 THI=1 (ug/m3)-1 y (mg/m3) y l mutagen Analyte CAS No. (ug/m3) (ug or fibers/m3) Acephate 30560-19-1 2.2E-06 I 9.0E-03 I V Acetaldehyde 75-07-0 5.6E+00 3.9E+01 Acetochlor 34256-82-1 3.1E+01 A V Acetone 67-64-1 1.4E+05 2.0E-03 X Acetone Cyanohydrin 75-86-5 8.8E+00 6.0E-02 I V Acetonitrile 75-05-8 2.6E+02 V Acetophenone 98-86-2 1.3E-03 C Acetylaminofluorene, 2- 53-96-3 9.4E-03 2.0E-05 I V Acrolein 107-02-8 8.8E-02 1.0E-04 I 6.0E-03 I M Acrylamide 79-06-1 1.2E-01 2.6E+01 1.0E-03 I V Acrylic Acid 79-10-7 4.4E+00 6.8E-05 I 2.0E-03 I V Acrylonitrile 107-13-1 1.8E-01 8.8E+00 6.0E-03 P Adiponitrile 111-69-3 2.6E+01 Alachlor 15972-60-8 Aldicarb 116-06-3 Aldicarb Sulfone 1646-88-4 Aldicarb sulfoxide 1646-87-3 4.9E-03 I V Aldrin 309-00-2 2.5E-03 1.0E-04 X V Allyl Alcohol 107-18-6 4.4E-01 6.0E-06 C 1.0E-03 I V Allyl Chloride 107-05-1 2.0E+00 4.4E+00 5.0E-03 P Aluminum 7429-90-5 2.2E+01 Aluminum Phosphide 20859-73-8 -

United States Patent Office Patented July 18, 1972 1
3,677,770 United States Patent Office Patented July 18, 1972 1. 2 It will be apparent that those fusible sugars which may 3,677,770 be employed have a melting or fusion point below their CARBONATED CANDY Frank Witzel, Spring Valley, N.Y., assignor to decomposition temperature, and that no substantial de Beech-Nut, Inc., New York, N.Y. composition occurs at the melting or fusion temperature No Drawing. Filed Oct. 7, 1970, Ser. No. 78,910 which would interfere with fusion, melting, or solidifica Int, Cl, A23g 3/00 tion on cooling. U.S. C. 99-134 R 2 Claims Although as will be apparent from this disclosure, the fusible sugars which may be used in the practice of this invention include those which have a melting or fusing ABSTRACT OF THE DISCLOSURE 0 point which falls within a wide range, the preferred mate rials will be those having a melting or fusing point at The off-taste present in effervescent hard candy due to temperatures of from slightly above room temperature unreacted food acidulant as well as salt formed by the to about 300 F. (149 C.). reaction of the efferverscent factors, i.e., leavening agent The fusible sugars which may be employed in the prac and acidulant, is overcome by incorporating a small tice of this invention include sugars and their derivatives amount of a saccharin into the candy. such as sugar alcohols and sugar acids. Typical fusible monosaccharide sugars include glucose, fructose (levu lose), invert sugar (chemically equal parts of glucose BACKGROUND OF THE INVENTION and fructose), arabinose, etc. -
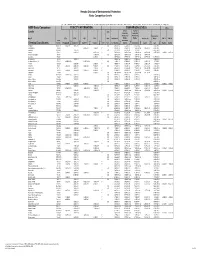
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V