Morphometric Comparison of Human Nerve Cells: Pyramidal Motor System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Questions on Human Anatomy
Standard Medical Text-books. ROBERTS’ PRACTICE OF MEDICINE. The Theory and Practice of Medicine. By Frederick T. Roberts, m.d. Third edi- tion. Octavo. Price, cloth, $6.00; leather, $7.00 Recommended at University of Pennsylvania. Long Island College Hospital, Yale and Harvard Colleges, Bishop’s College, Montreal; Uni- versity of Michigan, and over twenty other medical schools. MEIGS & PEPPER ON CHILDREN. A Practical Treatise on Diseases of Children. By J. Forsyth Meigs, m.d., and William Pepper, m.d. 7th edition. 8vo. Price, cloth, $6.00; leather, $7.00 Recommended at thirty-five of the principal medical colleges in the United States, including Bellevue Hospital, New York, University of Pennsylvania, and Long Island College Hospital. BIDDLE’S MATERIA MEDICA. Materia Medica, for the Use of Students and Physicians. By the late Prof. John B Biddle, m.d., Professor of Materia Medica in Jefferson Medical College, Phila- delphia. The Eighth edition. Octavo. Price, cloth, $4.00 Recommended in colleges in all parts of the UnitedStates. BYFORD ON WOMEN. The Diseases and Accidents Incident to Women. By Wm. H. Byford, m.d., Professor of Obstetrics and Diseases of Women and Children in the Chicago Medical College. Third edition, revised. 164 illus. Price, cloth, $5.00; leather, $6.00 “ Being particularly of use where questions of etiology and general treatment are concerned.”—American Journal of Obstetrics. CAZEAUX’S GREAT WORK ON OBSTETRICS. A practical Text-book on Midwifery. The most complete book now before the profession. Sixth edition, illus. Price, cloth, $6.00 ; leather, $7.00 Recommended at nearly fifty medical schools in the United States. -

Blepharoplasty
Blepharoplasty Bobby Tajudeen Brow position • medial brow as having its medial origin at the level of a vertical line drawn to the nasal alar-facial junction • lateral extent of the brow should reach a point on a line drawn from the nasal alar-facial junction through the lateral canthus of the eye • brow should arch superiorly, well above the supraorbital rim, with the highest point lying at the lateral limbus • Less arched in men • midpupillary line and the inferior brow border should be approximately 2.5 cm. The distance from the superior border of the brow to the anterior hairline should be 5 cm Eyelid aesthetics • The highest point of the upper eyelid is at the medial limbus, and the lowest point of the lower eyelid is at the lateral limbus. • Sharp canthal angles should exist, especially at the lateral canthus. • The upper eyelid orbicularis muscle should be smooth and flat, and the upper eyelid crease should be crisp. The upper lid crease should lie between 8 and 12 mm from the lid margin in the Caucasian patient. • The upper lid margin should cover 1 to 2 mm of the superior limbus, and the lower lid margin should lie at the inferior limbus or 1 mm below the inferior limbus • The lower eyelid should closely appose the globe without any drooping of the lid away from the globe (ectropion) or in toward the globe (entropion) Lid laxity and excess • A pinch test helps determine the degree of excess lid skin that is present. The snap test helps determine the degree of lower lid laxity and is useful in preoperative planning Evaluation • -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

Anatomy of the Periorbital Region Review Article Anatomia Da Região Periorbital
RevSurgicalV5N3Inglês_RevistaSurgical&CosmeticDermatol 21/01/14 17:54 Página 245 245 Anatomy of the periorbital region Review article Anatomia da região periorbital Authors: Eliandre Costa Palermo1 ABSTRACT A careful study of the anatomy of the orbit is very important for dermatologists, even for those who do not perform major surgical procedures. This is due to the high complexity of the structures involved in the dermatological procedures performed in this region. A 1 Dermatologist Physician, Lato sensu post- detailed knowledge of facial anatomy is what differentiates a qualified professional— graduate diploma in Dermatologic Surgery from the Faculdade de Medician whether in performing minimally invasive procedures (such as botulinum toxin and der- do ABC - Santo André (SP), Brazil mal fillings) or in conducting excisions of skin lesions—thereby avoiding complications and ensuring the best results, both aesthetically and correctively. The present review article focuses on the anatomy of the orbit and palpebral region and on the important structures related to the execution of dermatological procedures. Keywords: eyelids; anatomy; skin. RESU MO Um estudo cuidadoso da anatomia da órbita é muito importante para os dermatologistas, mesmo para os que não realizam grandes procedimentos cirúrgicos, devido à elevada complexidade de estruturas envolvidas nos procedimentos dermatológicos realizados nesta região. O conhecimento detalhado da anatomia facial é o que diferencia o profissional qualificado, seja na realização de procedimentos mini- mamente invasivos, como toxina botulínica e preenchimentos, seja nas exéreses de lesões dermatoló- Correspondence: Dr. Eliandre Costa Palermo gicas, evitando complicações e assegurando os melhores resultados, tanto estéticos quanto corretivos. Av. São Gualter, 615 Trataremos neste artigo da revisão da anatomia da região órbito-palpebral e das estruturas importan- Cep: 05455 000 Alto de Pinheiros—São tes correlacionadas à realização dos procedimentos dermatológicos. -

Double-Bellied Superior Rectus Muscle
Surgical and Radiologic Anatomy (2019) 41:713–715 https://doi.org/10.1007/s00276-019-02211-0 ANATOMIC VARIATIONS Double-bellied superior rectus muscle Satheesha B. Nayak1 · Surekha D. Shetty1 · Naveen Kumar1 · Ashwini P. Aithal1 Received: 3 September 2018 / Accepted: 23 February 2019 / Published online: 7 March 2019 © Springer-Verlag France SAS, part of Springer Nature 2019 Abstract Congenital variations of extraocular muscles are rare. We report a double-bellied superior rectus muscle, observed in an adult male cadaver aged 70 years. The superior rectus muscle had two equal-sized bellies, which took separate origins from the common tendinous ring and united to form a common belly 1 cm before the insertion. Due to the duplication, the muscle extended laterally beyond the levator palpebrae superioris. Both its bellies were supplied by oculomotor nerve. To the best of our knowledge, this is the first report on doubling of the belly of the superior rectus muscle. Keywords Extraocular · Orbit · Superior rectus muscle · Eye movement · Strabismus Introduction Case report Voluntary movements of the eyeball are performed by six During dissection classes for the first-year medical students, extraocular muscles, namely superior rectus muscle, the we observed a unique variation in the right orbit of an adult inferior rectus muscle, medial rectus muscle, lateral rectus male cadaver aged 70 years. The cadaver was donated to the muscle, superior oblique muscle, and inferior oblique mus- department for teaching and research purpose. No history of cles. Variations of these muscles can result in restrictions of strabismus or visual defects is available. The variation was movements of eyeball, causing strabismus. -

SŁOWNIK ANATOMICZNY (ANGIELSKO–Łacinsłownik Anatomiczny (Angielsko-Łacińsko-Polski)´ SKO–POLSKI)
ANATOMY WORDS (ENGLISH–LATIN–POLISH) SŁOWNIK ANATOMICZNY (ANGIELSKO–ŁACINSłownik anatomiczny (angielsko-łacińsko-polski)´ SKO–POLSKI) English – Je˛zyk angielski Latin – Łacina Polish – Je˛zyk polski Arteries – Te˛tnice accessory obturator artery arteria obturatoria accessoria tętnica zasłonowa dodatkowa acetabular branch ramus acetabularis gałąź panewkowa anterior basal segmental artery arteria segmentalis basalis anterior pulmonis tętnica segmentowa podstawna przednia (dextri et sinistri) płuca (prawego i lewego) anterior cecal artery arteria caecalis anterior tętnica kątnicza przednia anterior cerebral artery arteria cerebri anterior tętnica przednia mózgu anterior choroidal artery arteria choroidea anterior tętnica naczyniówkowa przednia anterior ciliary arteries arteriae ciliares anteriores tętnice rzęskowe przednie anterior circumflex humeral artery arteria circumflexa humeri anterior tętnica okalająca ramię przednia anterior communicating artery arteria communicans anterior tętnica łącząca przednia anterior conjunctival artery arteria conjunctivalis anterior tętnica spojówkowa przednia anterior ethmoidal artery arteria ethmoidalis anterior tętnica sitowa przednia anterior inferior cerebellar artery arteria anterior inferior cerebelli tętnica dolna przednia móżdżku anterior interosseous artery arteria interossea anterior tętnica międzykostna przednia anterior labial branches of deep external rami labiales anteriores arteriae pudendae gałęzie wargowe przednie tętnicy sromowej pudendal artery externae profundae zewnętrznej głębokiej -

A- and V-Patterns and Oblique Muscle Overaction A- and V-Patterns
18 A- and V-Patterns and Oblique Muscle Overaction A- and V-Patterns Clinical Features Etiology Management with Horizontal Rectus Muscle Offsets Inferior Oblique Overaction Etiology Clinical Features Differential Diagnosis Management Superior Oblique Overaction Etiology Clinical Features Differential Diagnosis Management Superior Oblique Weakening Procedures Complications A- and V-patterns of strabismus are changes in the horizontal deviation as the patient looks up and down. They are usually associated with oblique dysfunction, either overaction or paresis. Primary oblique muscle overaction is when an oblique muscle is too strong for its antagonist and there is no known cause. Secondary oblique overaction is caused by a paresis of the antagonist muscle. A superior oblique paresis results in inferior oblique overaction, and inferior oblique paresis results in superior oblique overaction. This chapter covers the management of A- and V- patterns and primary oblique muscle overaction. A- and V-Patterns CLINICAL FEATURES A-patterns are defined as increasing divergence in down gaze (>10 prism diopters [PD]), whereas Vpatterns are increased divergence (>15 prism diopters) in up gaze. The type of A- or V-pattern helps identify the cause. Superior oblique paresis produces a V-pattern, arrow subtype, with convergence in down gaze. The arrow pattern subtype indicates a lack of abduction in down gaze, the field of action of the superior oblique muscles. Inferior oblique overaction, on the other hand, has a V-pattern, Y subtype, with increased abduction in up gaze. The Y-pattern occurs because the field of action of the inferior oblique muscles is up gaze and they are abductors. Lambda subtype is typically associated with superior oblique overaction, with increased abduction in down gaze, because the field of action is in down gaze. -

Kinesiology of the Head and Spine
Oatis_CH20_389-411.qxd 4/18/07 3:10 PM Page 389 PART Kinesiology of the Head III and Spine Vertebral body Inferior articular process of superior vertebra Superior articular process of inferior vertebra Spinous process UNIT 4: MUSCULOSKELETAL FUNCTIONS WITHIN THE HEAD Chapter 20: Mechanics and Pathomechanics of the Muscles of the Face and Eyes Chapter 21: Mechanics and Pathomechanics of Vocalization Chapter 22: Mechanics and Pathomechanics of Swallowing Chapter 23: Structure and Function of the Articular Structures of the TMJ Chapter 24: Mechanics and Pathomechanics of the Muscles of the TMJ Chapter 25: Analysis of the Forces on the TMJ during Activity UNIT 5: SPINE UNIT Chapter 26: Structure and Function of the Bones and Joints of the Cervical Spine Chapter 27: Mechanics and Pathomechanics of the Cervical Musculature Chapter 28: Analysis of the Forces on the Cervical Spine during Activity Chapter 29: Structure and Function of the Bones and Joints of the Thoracic Spine Chapter 30: Mechanics and Pathomechanics of the Muscles of the Thoracic Spine Chapter 31: Loads Sustained by the Thoracic Spine Chapter 32: Structure and Function of the Bones and Joints of the Lumbar Spine Chapter 33: Mechanics and Pathomechanics of Muscles Acting on the Lumbar Spine Chapter 34: Analysis of the Forces on the Lumbar Spine during Activity Chapter 35: Structure and Function of the Bones and Joints of the Pelvis Chapter 36: Mechanics and Pathomechanics of Muscle Activity in the Pelvis Chapter 37: Analysis of the Forces on the Pelvis during Activity 389 Oatis_CH20_389-411.qxd 4/18/07 3:10 PM Page 390 PARTUNIT 4V MUSCULOSKELETAL FUNCTIONS WITHIN THE HEAD he preceding three units examine the structure, function, and dysfunction of the upper extremity, which is part of the appendicular skeleton. -
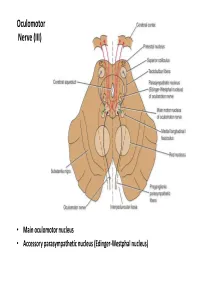
Cranial Nerves
Oculomotor Nerve (III) • Main oculomotor nucleus • Accessory parasympathetic nucleus (Edinger-Westphal nucleus) Course of occulomotor nerve • Red nucleus • Interpeduncular fossa • Middle cranial fossa in the lateral wall of the cavernous sinus (Two rami) • superior orbital fissure Oculomotor Nerve (III) • Extrinsic muscles: – The levator palpebrae superioris, superior rectus, medial rectus, inferior rectus, and inferior oblique • Intrinsic muscles: – The constrictor pupillae of the iris and ciliary muscles Action: Lifting the upper eyelid; turning the eye upward, downward, and medially; constricting the pupil; and accommodating the eye Oculomotor Nerve injury • Complete lesion – All of the muscles are paralyzed except lateral rectus and superior oblique – Symptoms: • External strabismus • Diplopia • Ptosis: drooping of the upper eyelid. • The pupil is widely dilated and nonreactive to light • Accommodation of the eye is paralyzed. Incomplete lesions: Internal ophthalmoplegia: loss of the autonomic innervation of the sphincter pupillae and ciliary muscle In cases of (diabetic neuropathy), the autonomic fibers are unaffected, whereas the nerves to the extraocular External ophthalmoplegia.: muscles are paralyzed. paralysis of the extraocular muscles Trochlear Nerve Nucleus • Location Pass posteriorly around the central gray matter Immediately decussates Trochlear Nerve • Supplies : superior oblique muscle • Action: turning the eye downward and laterally Trochlear Nerve injury • Symptoms: – Diplopia – Difficulty in turning the eye downward -

Atlas of Topographical and Pathotopographical Anatomy of The
Contents Cover Title page Copyright page About the Author Introduction Part 1: The Head Topographic Anatomy of the Head Cerebral Cranium Basis Cranii Interna The Brain Surgical Anatomy of Congenital Disorders Pathotopography of the Cerebral Part of the Head Facial Head Region The Lymphatic System of the Head Congenital Face Disorders Pathotopography of Facial Part of the Head Part 2: The Neck Topographic Anatomy of the Neck Fasciae, Superficial and Deep Cellular Spaces and their Relationship with Spaces Adjacent Regions (Fig. 37) Reflex Zones Triangles of the Neck Organs of the Neck (Fig. 50–51) Pathography of the Neck Topography of the neck Appendix A Appendix B End User License Agreement Guide 1. Cover 2. Copyright 3. Contents 4. Begin Reading List of Illustrations Chapter 1 Figure 1 Vessels and nerves of the head. Figure 2 Layers of the frontal-parietal-occipital area. Figure 3 Regio temporalis. Figure 4 Mastoid process with Shipo’s triangle. Figure 5 Inner cranium base. Figure 6 Medial section of head and neck Figure 7 Branches of trigeminal nerve Figure 8 Scheme of head skin innervation. Figure 9 Superficial head formations. Figure 10 Branches of the facial nerve Figure 11 Cerebral vessels. MRI. Figure 12 Cerebral vessels. Figure 13 Dural venous sinuses Figure 14 Dural venous sinuses. MRI. Figure 15 Dural venous sinuses Figure 16 Venous sinuses of the dura mater Figure 17 Bleeding in the brain due to rupture of the aneurism Figure 18 Types of intracranial hemorrhage Figure 19 Different types of brain hematomas Figure 20 Orbital muscles, vessels and nerves. Topdown view, Figure 21 Orbital muscles, vessels and nerves. -

Differences in Extrinsic Eye Muscles and Dissection Protocols
Iowa State University Capstones, Theses and Creative Components Dissertations Spring 2020 Comparative anatomy case study: differences in extrinsic eye muscles and dissection protocols Erin Ruden Follow this and additional works at: https://lib.dr.iastate.edu/creativecomponents Part of the Animal Structures Commons, and the Body Regions Commons Recommended Citation Ruden, Erin, "Comparative anatomy case study: differences in extrinsic eye muscles and dissection protocols" (2020). Creative Components. 543. https://lib.dr.iastate.edu/creativecomponents/543 This Creative Component is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Creative Components by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. P a g e | 1 Comparative anatomy case study: differences in extrinsic eye muscles and dissection protocols Abstract: There have been notable studies comparing the eye placement between predators and prey, which can be further categorized as omnivore, carnivore, and herbivore. The purpose of this study was to determine if eye placement correlated with extrinsic eye muscle differences among the classification of animals (predator vs. prey and omnivore vs. carnivore vs. herbivore). For an omnivore, a human orbit was dissected. A cat was dissected to represent a carnivore and a goat was dissected to represent an herbivore. Dissection protocol for the cat and goat were created due to the lack of dissections of these muscles. Unfortunately, data collection was incomplete due to the social distancing policy on Iowa State University’s campus because of COVID-19. -

Anatomy and Physiology Model Guide Book
Anatomy & Physiology Model Guide Book Last Updated: August 8, 2013 ii Table of Contents Tissues ........................................................................................................................................................... 7 The Bone (Somso QS 61) ........................................................................................................................... 7 Section of Skin (Somso KS 3 & KS4) .......................................................................................................... 8 Model of the Lymphatic System in the Human Body ............................................................................. 11 Bone Structure ........................................................................................................................................ 12 Skeletal System ........................................................................................................................................... 13 The Skull .................................................................................................................................................. 13 Artificial Exploded Human Skull (Somso QS 9)........................................................................................ 14 Skull ......................................................................................................................................................... 15 Auditory Ossicles ....................................................................................................................................