PRODUCT INFORMATION PRO-CID™ (Probenecid Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Familial Mediterranean Fever
:: Familial Mediterranean fever – This document is a translation of the French recommendations drafted by Prof Gilles Grateau, Dr Véronique Hentgen, Dr Katia Stankovic Stojanovic and Dr Gilles Bagou reviewed and published by Orphanet in 2010. – Some of the procedures mentioned, particularly drug treatments, may not be validated in the country where you practice. Synonyms: Periodic disease, FMF Definition: Familial Mediterranean fever (FMF) is an auto-inflammatory disease of genetic origin, affecting Mediterranean populations and characterised by recurrent attacks of fever accompanied by polyserositis that causes the symptoms. Colchicine is the basic reference treatment and is designed to tackle inflammatory attacks and prevent amyloidosis, the most severe complication of FMF. Further information: See the Orphanet abstract http://www.orpha.net/data/patho/Pro/en/Emergency_FamilialMediterraneanFever-enPro920.pdf ©Orphanet UK 1/5 Pre-hospital emergency care recommendations Call for a patient suffering from Familial mediterranean fever Synonyms periodic disease FMF Mechanisms auto-inflammatory disease that affects Mediterranean populations in particular, due to mutation of the MEFV gene that codes pyrin or marenostrin, this being the underlying cause of congenital immune dysfunction; repeated inflammatory attacks can lead to amyloidosis, particularly renal Specific risks in emergency situations acute inflammatory attack, particularly abdominal (pseudo-surgical), but also thoracic, articular (knees) or testicular fever as an expression -

Guidelines for Management of Gout. Part 1: Systematic Nonpharmacologic and Pharmacologic Therapeutic Approaches to Hyperuricemia DINESH KHANNA,1 JOHN D
Arthritis Care & Research Vol. 64, No. 10, October 2012, pp 1431–1446 DOI 10.1002/acr.21772 © 2012, American College of Rheumatology SPECIAL ARTICLE 2012 American College of Rheumatology Guidelines for Management of Gout. Part 1: Systematic Nonpharmacologic and Pharmacologic Therapeutic Approaches to Hyperuricemia DINESH KHANNA,1 JOHN D. FITZGERALD,2 PUJA P. KHANNA,1 SANGMEE BAE,2 MANJIT K. SINGH,3 TUHINA NEOGI,4 MICHAEL H. PILLINGER,5 JOAN MERILL,6 SUSAN LEE,7 SHRADDHA PRAKASH,2 MARIAN KALDAS,2 MANEESH GOGIA,2 FERNANDO PEREZ-RUIZ,8 WILL TAYLOR,9 FRE´ DE´ RIC LIOTE´ ,10 HYON CHOI,4 JASVINDER A. SINGH,11 NICOLA DALBETH,12 SANFORD KAPLAN,13 VANDANA NIYYAR,14 DANIELLE JONES,14 STEVEN A. YAROWS,15 BLAKE ROESSLER,1 GAIL KERR,16 CHARLES KING,17 GERALD LEVY,18 DANIEL E. FURST,2 N. LAWRENCE EDWARDS,19 BRIAN MANDELL,20 H. RALPH SCHUMACHER,21 MARK ROBBINS,22 2 7 NEIL WENGER, AND ROBERT TERKELTAUB Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology (ACR) are intended to provide guidance for particular patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to these guidelines and recommendations to be voluntary, with the ultimate determi- nation regarding their application to be made by the physician in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. Guidelines and recommendations developed or endorsed by the ACR are subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice. -

Colchicine and Probenecid | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Colchicine and Probenecid This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. What is this drug used for? It is used to prevent gouty arthritis. What do I need to tell my doctor BEFORE I take this drug? For all patients taking this drug: If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have any of these health problems: Blood problems or kidney stones. If you have any of these health problems: Kidney disease or liver disease. If you are pregnant or may be pregnant. Do not take this drug if you are pregnant. If you are taking a salicylate drug like aspirin. If you have liver or kidney problems and you take certain other drugs. Very bad and sometimes deadly side effects have happened in these people. There are many drugs that can do this. Ask your doctor or pharmacist if you are not sure. Children: If the patient is a child younger than 2 years of age. Do not give this drug to a child younger than 2 years of age. Colchicine and Probenecid 1/6 This is not a list of all drugs or health problems that interact with this drug. Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -

Gout Management in Swiss Primary Care – a Retrospective Observational Study
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Gout management in Swiss primary care - a retrospective observational study Meier, Rahel ; di Gangi, Stefania ; Valeri, Fabio ; Rosemann, Thomas ; Zechmann, Stefan Abstract: BACKGROUND Gout is the most common form of inflammatory arthritis worldwide and its prevalence is rising. In Switzerland, there are no data available on the characteristics and treat- ment of gout patients. In this study, we aimed to describe numbers of patients affected by gout and hyperuricaemia and unveil approaches Swiss primary care physicians (PCPs) use for the management. METHODS This was a retrospective observational study using electronic medical routine nbsp;data pro- vided from 242 Swiss PCPs. Included were all their patients receiving urate-lowering therapy (ULT), with a diagnostic code for gout or who had a serum uric acid (SUA) measurement. According to their disease status, patients were classified into four subgroups (normal urate, hyperuricaemia, untreated gout, treated gout). For treatment analysis, patients with SUA measurements before and after ULT initiation were in- cluded. Comorbidities and risk factors for secondary causes relevant in the context of gout were collected. Outcomes were prevalence of gout and hyperuricaemia, characteristics of patients according to subgroup, number of SUA measurements, levels of SUA and patients who reached the treatment goal of a SUA level lt;360 micro;mol/l. RESULTS We assessed 15,808 patients and classified them into the subgroups. This yielded a prevalence of 1.0% for gout and 1.2% for hyperuricaemia. 2642 patients were diagnosed with gout of whom 2420 (91.6%) received a ULT. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

The Effect of Caffeine and Trifluralin on Chromosome Doubling in Wheat
plants Article The Effect of Caffeine and Trifluralin on Chromosome Doubling in Wheat Anther Culture Sue Broughton *, Marieclaire Castello, Li Liu, Julie Killen, Anna Hepworth and Rebecca O’Leary Department of Primary Industries and Regional Development, 3 Baron-Hay Court, South Perth 6151, Western Australia, Australia; [email protected] (M.C.); [email protected] (L.L.); [email protected] (J.K.); [email protected] (A.H.); Rebecca.O'[email protected] (R.O.) * Correspondence: [email protected] Received: 3 December 2019; Accepted: 10 January 2020; Published: 15 January 2020 Abstract: Challenges for wheat doubled haploid (DH) production using anther culture include genotype variability in green plant regeneration and spontaneous chromosome doubling. The frequency of chromosome doubling in our program can vary from 14% to 80%. Caffeine or trifluralin was applied at the start of the induction phase to improve early genome doubling. Caffeine treatment at 0.5 mM for 24 h significantly improved green plant production in two of the six spring wheat crosses but had no effect on the other crosses. The improvements were observed in Trojan/Havoc and Lancer/LPB14-0392, where green plant numbers increased by 14% and 27% to 161 and 42 green plants per 30 anthers, respectively. Caffeine had no significant effect on chromosome doubling, despite a higher frequency of doubling in several caffeine treatments in the first experiment (67–68%) compared to the control (56%). In contrast, trifluralin significantly improved doubling following a 48 h treatment, from 38% in the control to 51% and 53% in the 1 µM and 3 µM trifluralin treatments, respectively. -

Your 2017 Three-Tier
Your 2017 Three-Tier Prescription Drug List effective January 1, 2017 Connecticut Advantage Three-Tier Please read: This document contains information about commonly prescribed medications. This Prescription Drug List (PDL) is accurate as of January 1, 2017 and is subject to change after this date. The next anticipated update will be in July 2017. Your estimated coverage and copay/co-insurance may vary based on the benefit plan you choose and the effective date of the plan. For additional information: Call the toll-free member phone number on your health plan ID card. Visit myuhc.com® • Locate a participating retail pharmacy by ZIP code. • Look up possible lower-cost medication alternatives. • Compare medication pricing and options. 1 Your Prescription Drug List This Prescription Drug List (PDL) outlines covered medications for certain conditions and organizes them into cost levels, also known as tiers. An important part of the PDL is giving you choices so you and your doctor can choose the best course of treatment for you. Go to myuhc.com® for complete drug information Since the PDL may change, we encourage you to visit our website, myuhc.com. This website is the best source for up-to-date information about the medications your pharmacy benefit covers, possible lower-cost options, and cost comparisons. To view PDL information without logging on to the member portal, visit myuhc.com and select “Pharmacy Information” under “Links and Tools.” 2 At UnitedHealthcare, we want to help you better understand your medication options. Your pharmacy benefit offers flexibility and choice in determining the right medication for you. -
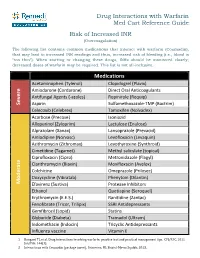
Drug Interactions with Warfarin Med Cart Reference Guide Risk of Increased INR (Overcoagulation)
Drug Interactions with Warfarin Med Cart Reference Guide Risk of Increased INR (Overcoagulation) The following list contains common medications that interact with warfarin (Coumadin), that may lead to increased INR readings and thus, increased risk of bleeding (i.e., blood is “too thin”). When starting or changing these drugs, INRs should be monitored closely; decreased doses of warfarin may be required. This list is not all-inclusive. Medications Acetaminophen (Tylenol) Clopidogrel (Plavix) Amiodarone (Cordarone) Direct Oral Anticoagulants Antifungal Agents (‐azoles) Ropinirole (Requip) Severe Aspirin Sulfamethoxazole‐TMP (Bactrim) Celecoxib (Celebrex) Tamoxifen (Nolvadex) Acarbose (Precose) Isoniazid Allopurinol (Zyloprim) Lactulose (Enulose) Alprazolam (Xanax) Lansoprazole (Prevacid) Amlodipine (Norvasc) Levofloxacin (Levaquin) Azithromycin (Zithromax) Levothyroxine (Synthroid) Cimetidine (Tagamet) Methyl salicylate (topical) Ciprofloxacin (Cipro) Metronidazole (Flagyl) Clarithromycin (Biaxin) Moxifloxacin (Avelox) Colchicine Omeprazole (Prilosec) Doxycycline (Vibratab) Phenytoin (Dilantin) Moderate Efavirenz (Sustiva) Protease Inhibitors Ethanol Quetiapine (Seroquel) Erythromycin (E.E.S.) Ranitidine (Zantac) Fenofibrate (Tricor, Trilipix) SSRI Antidepressants Gemfibrozil (Lopid) Statins Glyburide (Diabeta) Tramadol (Ultram) Indomethacin (Indocin) Tricyclic Antidepressants Influenza vaccine Vitamin E 1. Bungard TJ, et al. Drug interactions involving warfarin: practice tool and practical management tips. CPJ/RPC. 2011 Jan/Feb. 144(1). 2. Interactions with Coumadin [package insert]. Princeton, NJ: Bristol‐Myers Squibb; 2013. Drug Interactions with Warfarin Med Cart Reference Guide Risk of Decreased INR (Undercoagulation) The following list contains common medications that interact with warfarin (Coumadin), which may lead to decreased INR readings and thus, increased risk of clotting, strokes, and DVTs (i.e., blood is “not thin enough”). When starting or changing these drugs, INRs should be monitored closely; increased doses of warfarin may be required. -

Discontinuation of Urate-Lowering Drugs: a Systematic Review Protocol
Discontinuation of urate-lowering drugs: a systematic review protocol Virginie Beslon, Perrine Moreau, Annabel Maruani, Hubert Maisonneuve, Bruno Giraudeau, Jean-Pascal Fournier ADMINISTRATIVE INFORMATION Registration PROSPERO registration number: Review team Virginie Beslon, Département de médecine générale, Université de Nantes, France ; [email protected] Perrine Moreau, Département de médecine générale, Université de Nantes, France ; [email protected] Annabel Maruani, Services de dermatologie, CHRU de Tours, Université François Rabelais, Tours, France ; [email protected] Hubert Maisonneuve, Unité des Internistes Généralistes et Pédiatres, Faculté de Médecine, Université de Genève, Suisse ; [email protected] Bruno Giraudeau, INSERM CIC 1415, CHRU de Tours, Université François-Rabelais, Tours, France ; [email protected] Jean-Pascal Fournier, Département de médecine générale, EA 4275-SPHERE, Université de Nantes, France ; [email protected] Contact details Jean-Pascal Fournier (guarantor) Département de médecine générale, Université de Nantes EA 4275-SPHERE, Biostatistique, Recherche Clinique et Mesures Subjectives en Santé, Université de Nantes 1 rue, Gaston Veil, 44000, Nantes, France [email protected] Contributions 1. Draft the protocol: VB, PM, AM, HM, BG, JPF 2. Study selection: VB, PM, JPF 3. Extract data from studies: VB, PM, JPF 4. Carry out the analysis: VB, PM, JPF 5. Interpret the analysis: VB, PM, AM, HM, BG, JPF 6. Draft the final review: VB, PM, JPF 7. Critical revision of the review for important intellectual content: VB, PM, AM, HM, BG, JPF Anticipated or actual start date December 30, 2015 Anticipated completion date October 30, 2016 Dissemination plans This paper will be submitted to a leading journal in this field. -

Drugs Which Can Affect Near Vision: a Useful List
Drugs Which Can Affect Near Vision: A Useful List Joanne L. Smith B.Sc., Ph.Phm.* J. Raymond Buncic, M.D., F.R.C.S.(C)t ABSTRACT This paper documents a list of drugs that cause problems with near vision, by virtue of effects on accommodation, occasionally refractive error and diplopia. It is meant as a reference aid to the clinician when confronted with problems of focusing on near objects or print. There are many drugs that have been reported to interfere with near or reading vision, producing blurring, decreased accommodation and diplopia. This paper lists the drugs that have been reported in the literature to produce symptoms which interfere with near vision. Case reports for the listed drugs vary greatly from many to few. The drugs have been divided into the following categories: those causing (A) blurring at near, (B) diplopia and (C) induced myopia. Those drugs which only rarely cause these symptoms have been omitted. From the Departments of Pharmacy* and Ophthalmologyt, The Hospital For Sick Children, Toronto, Ontario, Canada Requests for reprints should be addressed to: Dr. J. Raymond Buncic, Department of Ophthalmology, The Hospital For Sick Children, 555 University Ave., Toronto, Ontario, Canada M5G lX8 TABLE 1 DRUGS COMMONLY CAUSING DIFFICULTY WITH FOCUSING AT NEAR OR BLURRED VISION. DRUG INCIDENCE REFERENCE Antipsychotics Chlorpromazine 14-23 8 Clozapine 5 8,14 Fluphenazine 1.2-4.3 8 Haloperidol 6.8-16 8 Loxapine 12,14 Perphenazine 7.4-17.8 8 Pimozide 20 8 Risperidone 1-2%, >/= 10% 11 Thioridazine 0.6-18 8 Thiothixene 20 8 -

Life-Threatening Colchicine Drug Interactions John R
drugINTERACTIONS: insights and observations Life-threatening Colchicine Drug Interactions John R. Horn, PharmD, FCCP,and Philip D. Hansten, PharmD after starting clarithromycin. He subse- Table Drs. Horn and Hansten are both pro- quently developed multiorgan failure Selected Drugs That Inhibit fessors of pharmacy at the University and died.1 Another patient on col- of Washington School of Pharmacy. P-glycoprotein chicine developed fever, diarrhea, and For an electronic version of this arti- Amiodarone Nicardipine abdominal pain 3 days after starting cle, including references if any, visit Clarithromycin Propafenone clarithromycin.2 He went on to develop www.hanstenandhorn.com. Cyclosporine Quinidine dehydration, pancytopenia, and acido- Diltiazem Ritonavir sis, but he survived. Two other patients Erythromycin Saquinavir ew drugs have been around as on colchicine died from agranulocyto- Indinavir Tacrolimus long as colchicine—it was men- sis when clarithromycin was given con- Itraconazole Tamoxifen tioned at least as far back as currently.3 F Ketoconazole Verapamil the time of Nero. Colchicine can be Similar life-threatening reactions Nelfinavir very effective in the treatment of gout have been reported in patients on and familial Mediterranean fever. Un- colchicine who received concomitant fortunately, however, its therapeutic therapy with other PGP inhibitors.4-7 In Managing Interactions effects (primarily on white blood cells) most cases, colchicine toxicity has Colchicine should not be used with can lead to life-threatening toxicity if occurred in patients on chronic clarithromycin or erythromycin, and colchicine plasma concentrations be- colchicine therapy who are then start- given the potential for fatal outcomes, come too high. One of the causes of ed on a PGP inhibitor.