GSK Annual Report & Accounts 2006
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNITED STATES SECURITIES and EXCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 SCHEDULE 13D Under the Securities Exchange Act of 1934 (Amendment No. 2)* GENOCEA BIOSCIENCES, INC. (Name of Issuer) Common Stock, Par Value $0.001 (Title of Class of Securities) 372427 10 4 (CUSIP Number) Victoria A. Whyte GlaxoSmithKline plc 980 Great West Road Brentford, Middlesex TW8 9GS England Telephone: +44 (0)208 047 5000 Name, Address and Telephone Number of Person Authorized to Receive Notices and Communications) July 30, 2015 (Date of Event which Requires Filing of this Statement) If the filing person has previously filed a statement on Schedule 13G to report the acquisition that is the subject of this Schedule 13D, and is filing this schedule because of §§240.13d-1(e), 240.13d-1(f) or 240.13d-1(g), check the following box. ☐ Note: Schedules filed in paper format shall include a signed original and five copies of the schedule, including all exhibits. See Rule.13d-7 for other parties to whom copies are to be sent. * The remainder of this cover page shall be filled out for a reporting person’s initial filing on this form with respect to the subject class of securities, and for any subsequent amendment containing information which would alter disclosures provided in a prior cover page. The information required on the remainder of this cover page shall not be deemed to be “filed” for the purpose of Section 18 of the Securities Exchange Act of 1934 (“Act”) or otherwise subject to the liabilities of that section of the Act but shall be subject to all other provisions of the Act (however, see the Notes). -

Governance and Remuneration 2014
Governance & remuneration reportStrategic In this section Our Board 72 Our Corporate Executive Team 76 Chairman’s letter 78 Corporate governance framework 79 Board report to shareholders Oversight and stewardship in 2014 and future actions 80 remuneration & Governance Leadership and effectiveness 82 Committee reports Audit & Risk 86 Nominations 92 Corporate Responsibility 94 Remuneration report Chairman’s annual statement 96 Annual report on remuneration 97 2014 Remuneration policy report 119 Financial statements Financial Investor information Investor GSK Annual Report 2014 71 Our Board Strategic reportStrategic Diversity Experience International experience Composition Tenure (Non-Executives) % % % % Scientific 19 Global 75 Executive 19 Up to 3 years 39 % % % % Finance 31 USA 100 Non-Executive 81 3-6 years 15 % % % % Industry 50 Europe 94 Male 69 7-9 years 23 % % % EMAP 63 Female 31 Over 9 years 23 Sir Christopher Gent 66 Skills and experience Chairman Sir Christopher has many years of experience of leading global businesses and a track record of delivering outstanding performance Governance & remuneration & Governance Nationality in highly competitive industries. He was appointed Managing Director British of Vodafone plc in 1985 and then became its Chief Executive Officer Appointment date in 1997 until his retirement in 2003. Sir Christopher was also a 1 June 2004 and as Chairman Non-Executive Director of Ferrari SpA and a member of the British on 1 January 2005 Airways International Business Advisory Board. Committee membership External appointments Corporate Responsibility Sir Christopher is a Senior Adviser at Bain & Co. Committee Chairman, Nominations, Remuneration and Finance Sir Philip Hampton 61 Skills and experience Chairman Designate Prior to joining GSK, Sir Philip chaired major FTSE 100 companies including J Sainsbury plc. -

The Everyday Lives of Recovering Heroin Users
The everyday lives of recovering heroin users Joanne Neale Sarah Nettleton Lucy Pickering The everyday lives of recovering heroin users The everyday lives of recovering heroin users Joanne Neale Sarah Nettleton Lucy Pickering Joanne Neale Professor of Public Health Faculty of Health & Life Sciences Oxford Brookes University Jack Straw’s Lane Marston Oxford OX3 0FL Email: [email protected] Sarah Nettleton Reader in Sociology Department of Sociology Wentworth College University of York Heslington York YO10 5DD Email: [email protected] Lucy Pickering Lecturer in Anthropology School of Social and Political Sciences University of Glasgow Adam Smith Building Glasgow G12 8RT Email: [email protected] Published by the RSA 8 John Adam Street London WC2N 6EZ +44 (0)20 7930 5115 Registered as a charity in England and Wales no. 212424 and in Scotland no. SCO 37784 Copyright © Joanne Neale 2012 The RSA is an enlightenment organisation devoted to finding innovative and practical solutions to today’s pressing social problems Cover photo: ‘Wings of butterfly,’ John Foxx. © Thinkstock Designed by Soapbox, www.soapbox.co.uk Printed and bound by CPI Antony Rowe www.theRSA.org Contents Acknowledgements 9 Foreword 10 Chapter 1: Setting the scene 14 Why read this book? 14 Why focus on recovery? 15 What is recovery? 15 A study of the everyday lives of recovering heroin users 17 Structure of the book 18 Chapter 2: Considering recovery 20 Introduction 20 Mapping services and support 20 Factors that can encourage and sustain recovery efforts -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -

Annual Review 2005
GS2184_Review_A\W2.qxd 7/3/06 4:58 pm Page fc1 Annual Review 2005 human being Do more, feel better, live longer GS2184_Review_A\W2.qxd 9/3/06 1:28 pm Page ifc2 01 An interview with Sir Christopher Gent, Chairman, and JP Garnier, Chief Executive Officer 05 Tachi Yamada, Chairman, Research & Development, Pharmaceuticals 06 Jean Stéphenne, President and General Manager, GSK Biologicals 08 John Clarke, President, Consumer Healthcare 11 David Stout, President, Pharmaceutical Operations 14 Performance highlights 15 Business operating review 18 The Board 19 The Corporate Executive Team 20 Summary remuneration report 23 Corporate governance 24 Responsibility statements 25 Summary financial statements 26 Summary information under US GAAP 27 Shareholder information 29 Chairman and CEO’s closing letter JP Garnier (left) and Sir Christopher Gent (right) GS2184_Review_A\W2.qxd 7/3/06 5:01 pm Page 01 “Discovering important medicines, eradicating diseases, improving the quality of people’s lives and making medicines available to a greater number of people. This is what we do – and what we do matters to people.” JP Garnier, Chief Executive Officer An interview with Sir Christopher Gent, Chairman and JP Garnier, Chief Executive Officer 2005: a year of success and progress “Thanks to the efforts of our employees around the company’s pipeline is one of the largest and most world, 2005 was a very successful year for GSK,” says promising in the industry, with 149 projects in clinical JP Garnier, Chief Executive Officer. “Not only was it development (as at the end of February 2006), our best year ever from a financial standpoint, we also including 95 new chemical entities (NCEs), 29 product made substantial progress with our pipeline of line extensions (PLEs) and 25 vaccines. -

2005 GSK Corporate Responsibility Report
2005 GSK Corporate Responsibility Report Introduction 1 Introduction Page Our contribution to society 1 About this report 1 CEO and Chairman’s letter 3 Managing corporate responsibility 4 Why CR is important to GSK 4 CR governance 4 Our CR principles 6 Stakeholder engagement 7 Stakeholder feedback 7 How we are responding 9 Government and external affairs 10 Membership of trade associations 10 US lobbying expenditures 10 Political donations 10 Our position on key issues 11 Patient advocacy 11 2 Access to medicine 3 Research 4 Ethical conduct 5 Employment practices 6 Human rights 7 Environment 8 Community investment 9 Data summary 1 1 GSK CORPORATE RESPONSIBILITY REPORT 2005 Introduction Welcome to GSK’s Corporate Responsibility Report 2005. This report explains our approach to the wide range of social, ethical and environmental issues associated with our business and reports our performance in 2005. The full web-based report is available on www.gsk.com GSK is a research-based pharmaceutical company, with operations in 119 countries. We make prescription medicines, vaccines, over-the-counter medicines, and consumer healthcare products. Our business accounts for 6.3% of the world’s pharmaceutical market. We have strong positions in several therapeutic areas including anti-infectives, asthma, cancer, cardiovascular, depression, diabetes, HIV/AIDS and urology. For an overview of our business, see our Annual Report. OUR CONTRIBUTION TO SOCIETY This report explains our approach to the significant In the last 80 years, medicines and vaccines have corporate responsibility issues for our business, including: transformed millions of lives. They have helped to increase • Access to medicines – how we make our medicines life expectancy and lowered death rates from conditions accessible to poor patients in developed and developing such as heart disease, stroke and cancer. -

In This Section
Strategic report In this section Chairman’s statement 2 CEO’s review 4 Business overview 6 The global context 8 Our business model 12 Our strategic priorities 14 How we performed 16 Risk management 18 Grow 20 Deliver 32 Simplify 44 Our financial architecture 48 Responsible business 50 Financial review 58 Strategic report Chairman’s statement Chairman’s statement To shareholders The value of the significant changes that have been made in recent years is evidenced in our performance this year “ Since Sir Andrew became It is clear from the following pages that Through the Audit & Risk Committee, we the Group made good progress against oversee the issues and challenges faced by CEO, the company has its strategy in 2013. management, and encourage the creation of an environment in which GSK can achieve The Board believes the business is seeing returned £30 billion its strategic ambitions in a responsible and the benefits of the significant changes the sustainable manner. to shareholders.” management team has driven over recent years to deliver sustainable growth, reduce risk and I have no doubt that commercial success is enhance returns to shareholders. directly linked to operating in a responsible way and which meets the changing expectations of The notably strong performance from the society. In this respect, the company continues R&D organisation in 2013 – with six major to adopt industry-leading positions on a range new product approvals in areas including of issues. respiratory disease, HIV and cancer – is critical to the longer-term prospects of the The announcement of plans during 2013 to Group. -
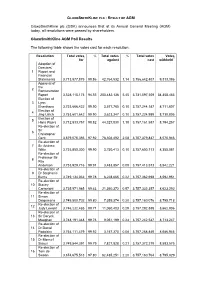
Voting/Poll Results
GLAXOSMITHKLINE PLC - RESULT OF AGM GlaxoSmithKline plc (GSK) announces that at its Annual General Meeting (AGM) today, all resolutions were passed by shareholders. GlaxoSmithKline AGM Poll Results The following table shows the votes cast for each resolution: Resolution Total votes % Total votes % Total votes Votes for* against cast withheld** Adoption of Directors’ 1 Report and Financial Statements 3,713,877,875 98.86 42,764,532 1.14 3,756,642,407 9,313,386 Approval of the 2 Remuneration Report 3,528,115,173 94.55 203,482,136 5.45 3,731,597,309 34,358,483 Election of 3 Lynn Elsenhans 3,753,666,422 99.90 3,577,765 0.10 3,757,244,187 8,711,607 Election of 4 Jing Ulrich 3,753,601,642 99.90 3,623,347 0.10 3,757,224,989 8,730,805 Election of 5 Hans Wijers 3,712,833,757 98.82 44,327,830 1.18 3,757,161,587 8,794,257 Re-election of Sir 6 Christopher Gent 3,679,075,355 97.92 78,304,492 2.08 3,757,379,847 8,575,946 Re-election of 7 Sir Andrew Witty 3,753,850,300 99.90 3,750,413 0.10 3,757,600,713 8,355,081 Re-election of Professor Sir 8 Roy Anderson 3,753,929,716 99.91 3,483,857 0.09 3,757,413,573 8,542,221 Re-election of 9 Dr Stephanie Burns 3,749,134,033 99.78 8,228,665 0.22 3,757,362,698 8,592,951 Re-election of 10 Stacey Cartwright 3,735,971,985 99.43 21,360,372 0.57 3,757,332,357 8,623,292 Re-election of 11 Simon Dingemans 3,749,800,702 99.80 7,359,374 0.20 3,757,160,076 8,795,718 Re-election of 12 Judy Lewent 3,746,232,485 99.71 11,060,403 0.29 3,757,292,888 8,662,906 Re-election of 13 Sir Deryck Maughan 3,748,191,348 99.76 9,051,199 0.24 -

Glaxosmithkline Plc Annual Report for the Year Ended 31St December 2000
GlaxoSmithKline 01 GlaxoSmithKline plc Annual Report for the year ended 31st December 2000 Contents Report of the Directors 02 Financial summary 03 Joint statement by the Chairman and the Chief Executive Officer 05 Description of business 29 Corporate governance 37 Remuneration report 47 Operating and financial review and prospects 69 Financial statements 70 Directors’ statements of responsibility 71 Report by the auditors 72 Consolidated statement of profit and loss 72 Consolidated statement of total recognised gains and losses 74 Consolidated statement of cash flow 76 Consolidated balance sheet 76 Reconciliation of movements in equity shareholders’ funds 77 Company balance sheet 78 Notes to the financial statements 136 Group companies 142 Principal financial statements in US$ 144 Financial record 153 Investor information 154 Shareholder return 156 Taxation information for shareholders 157 Shareholder information 158 Share capital 160 Cross reference to Form 20-F 162 Glossary of terms The Annual Report was approved by the Board 163 Index of Directors on 22nd March 2001 and published on 12th April 2001. Contact details 02 GlaxoSmithKline Financial summary 2000 1999 Increase Business performance £m £m CER % £ % Sales 18,079 16,164 9 12 Trading profit 5,026 4,378 12 15 Profit before taxation 5,327 4,708 11 13 Earnings/Net income 3,697 3,222 13 15 Earnings per Ordinary Share 61.0p 52.7p 14 16 Total results Profit before taxation 6,029 4,236 Earnings/Net income 4,154 2,859 Earnings per Ordinary Share 68.5p 46.7p Business performance: results exclude merger items and restructuring costs; 1999 sales and trading profit exclude the Healthcare Services businesses which were disposed of in 1999. -

Corporate Responsibility Report 2008
Corporate Responsibility Report 2008 Strength through responsibility Summary Reed Elsevier Corporate Responsibility Report 2008 Chief Executive’s introduction Governance People Our true performance as a company must take account of both our non-financial and financial results. They are intrinsically linked – if we want to be a profitable and successful company, we must be an ethical one. We are committed to being both. To that end, we have been building on our expertise and engaging with stakeholders to find ways to innovate and improve. Health and safety In developing online solutions to the needs of our customers in areas like health, science and technology, law, environment, and business, we benefit society. For example, Procedures Consult gives doctors a way of maintaining their skills and knowledge through web-based simulation of essential medical techniques; TotalPatent is a single source for global patents, a primary driver in research and development; XpertHR helps practitioners advance good practice in people management; energylocate aggregates our energy offerings into a one-stop community, using tools like social networking and video to advance knowledge. Customers Innovation is also helping advance our internal corporate responsibility: > Increased online training in our Code of Ethics and Business Conduct has led to more of our people understanding what it means to do the right thing > A new global jobs board illuminates our value, Boundarylessness, by allowing employees to search for new positions across locations, functions, -

22 November 2012 • No 5007 • Vol 143 Gazette
ThursdaY 22 november 2012 • no 5007 • vol 143 Gazette Council and Main Notices 179 Advertisements 183 Committees 176 Consultative notices: Council of the University: Consolidation of small trust funds Notifications of Vacancies 186 Approval of nomination of external member of Council General notices: University of Oxford Gazette publication arrangements Council of the University: Colleges, Halls and Societies Changes in Regulations: Appointments: External Vacancies (a) Committees reporting directly to University Administration and Council or one of its main Services Supplement included with this issue: committees (1) to no 5006: Topic for discussion: (b) Regulations for the Student Visiting Professorships: the libraries and their future 159–174 Fitness to Study Panel Medical Sciences (c) Delegacy for Military Instruction (d) Personnel Committee Electoral Boards: (e) Research Committee Professorship of Economics (f) Socially Responsible Investment Newton Abraham Visiting Review Committee Professorship in the Medical, (g) Income grant from the College Biological and Chemical Sciences Contributions Fund Musical and other Events: General Purposes Committee of Council: lincoln Changes in Regulations: Exhibitions: Saïd Business School and Business Magdalen Advisory Council Council of the University: Lectures 181 Register of Congregation Examinations and Boards 181 Congregation 178 Examinations for the Degree of Doctor of Congregation 26 November: Philosophy Degree by Resolution Examinations for the Degree of Master of Congregation 27 november: -

Vote Summary
Vote Summary FENNER PLC, HESSLE YORKSHIRE Security G33656102 Meeting Type Annual General Meeting Ticker Symbol Meeting Date 14-Jan-2015 ISIN GB0003345054 Agenda 705747369 - Management Record Date Holding Recon Date 12-Jan-2015 City / Country LONDON / United Vote Deadline Date 08-Jan-2015 Kingdom SEDOL(s) 0334505 - B3BH6Q8 Quick Code Proposal Proposed Vote For/Against by Manageme nt Item 1 TO RECEIVE THE REPORTS OF THE DIRECTORS Management For For 2 TO APPROVE THE BOARD REMUNERATION POLICY Management For For 3 TO APPROVE THE BOARD ANNUAL REMUNERATION Management For For 4 TO DECLARE A DIVIDEND Management For For 5 TO RE-ELECT MARK ABRAHAMS Management For For 6 TO RE-ELECT NICHOLAS HOBSON Management For For 7 TO RE-ELECT RICHARD PERRY Management For For 8 TO RE-ELECT VANDA MURRAY Management For For 9 TO RE-ELECT JOHN SHELDRICK Management For For 10 TO RE-ELECT ALAN WOOD Management For For 11 TO RE-APPOINT THE AUDITORS Management For For 12 TO AUTHORISE THE DIRECTORS TO DETERMINE Management For For 13 TO APPROVE THE FENNER PERFORMANCE SHARE Management For For 14 AUTHORITY TO ALLOT SHARES Management For For 15 POWER TO ALLOT SHARES FOR CASH AND Management For For 16 AUTHORITY TO BUY BACK SHARES Management For For 17 TO ALLOW THE COMPANY TO HOLD GENERAL Management For For IMPERIAL BRANDS PLC, BRISTOL Security G4721W102 Meeting Type Annual General Meeting Ticker Symbol Meeting Date 28-Jan-2015 ISIN GB0004544929 Agenda 705751356 - Management Record Date Holding Recon Date 26-Jan-2015 City / Country BRISTOL / United Vote Deadline Date 22-Jan-2015