A Novel Type of Gabaergic Interneuron Connecting the Input and the Output Regions of the Hippocampus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Glutamate Binding in Primate Brain
Journal of Neuroscience Research 27512-521 (1990) Quisqualate- and NMDA-Sensitive [3H]Glutamate Binding in Primate Brain A.B. Young, G.W. Dauth, Z. Hollingsworth, J.B. Penney, K. Kaatz, and S. Gilman Department of Neurology. University of Michigan, Ann Arbor Excitatory amino acids (EAA) such as glutamate and Young and Fagg, 1990). Because EAA are involved in aspartate are probably the neurotransmitters of a general cellular metabolic functions, they were not orig- majority of mammalian neurons. Only a few previous inally considered to be likcl y neurotransmitter candi- studies have been concerned with the distribution of dates. Electrophysiological studies demonstrated con- the subtypes of EAA receptor binding in the primate vincingly the potency of these substances as depolarizing brain. We examined NMDA- and quisqualate-sensi- agents and eventually discerned specific subtypes of tive [3H]glutamate binding using quantitative autora- EAA receptors that responded preferentially to EAA an- diography in monkey brain (Macaca fascicularis). alogs (Dingledine et al., 1988). Coincident with the elec- The two types of binding were differentially distrib- trophysiological studies, biochemical studies indicated uted. NMDA-sensitive binding was most dense in that EAA were released from slice and synaptosome dentate gyrus of hippocampus, stratum pyramidale preparations in a calcium-dependent fashion and were of hippocampus, and outer layers of cerebral cortex. accumulated in synaptosomc preparations by a high-af- Quisqualate-sensitive binding was most dense in den- finity transport system. With these methodologies, EAA tate gyrus of hippocampus, inner and outer layers of release and uptake were found to be selectively de- cerebral cortex, and molecular layer of cerebellum. -

Innovations Present in the Primate Interneuron Repertoire
Article Innovations present in the primate interneuron repertoire https://doi.org/10.1038/s41586-020-2781-z Fenna M. Krienen1,2 ✉, Melissa Goldman1,2, Qiangge Zhang2,3, Ricardo C. H. del Rosario2, Marta Florio1,2, Robert Machold4, Arpiar Saunders1,2, Kirsten Levandowski2,3, Heather Zaniewski2,3, Received: 19 July 2019 Benjamin Schuman4, Carolyn Wu3, Alyssa Lutservitz1,2, Christopher D. Mullally1,2, Nora Reed1,2, Accepted: 1 July 2020 Elizabeth Bien1,2, Laura Bortolin1,2, Marian Fernandez-Otero2,5, Jessica D. Lin2, Alec Wysoker2, James Nemesh2, David Kulp2, Monika Burns5, Victor Tkachev6,7,8, Richard Smith9,10, Published online: xx xx xxxx Christopher A. Walsh9,10, Jordane Dimidschstein2, Bernardo Rudy4,11, Leslie S. Kean6,7,8, Check for updates Sabina Berretta5,12,13, Gord Fishell2,14, Guoping Feng2,3 & Steven A. McCarroll1,2 ✉ Primates and rodents, which descended from a common ancestor around 90 million years ago1, exhibit profound diferences in behaviour and cognitive capacity; the cellular basis for these diferences is unknown. Here we use single-nucleus RNA sequencing to profle RNA expression in 188,776 individual interneurons across homologous brain regions from three primates (human, macaque and marmoset), a rodent (mouse) and a weasel (ferret). Homologous interneuron types—which were readily identifed by their RNA-expression patterns—varied in abundance and RNA expression among ferrets, mice and primates, but varied less among primates. Only a modest fraction of the genes identifed as ‘markers’ of specifc interneuron subtypes in any one species had this property in another species. In the primate neocortex, dozens of genes showed spatial expression gradients among interneurons of the same type, which suggests that regional variation in cortical contexts shapes the RNA expression patterns of adult neocortical interneurons. -

Distinct Transcriptomic Cell Types and Neural Circuits of the Subiculum and Prosubiculum Along 2 the Dorsal-Ventral Axis 3 4 Song-Lin Ding1,2,*, Zizhen Yao1, Karla E
bioRxiv preprint doi: https://doi.org/10.1101/2019.12.14.876516; this version posted December 15, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Distinct transcriptomic cell types and neural circuits of the subiculum and prosubiculum along 2 the dorsal-ventral axis 3 4 Song-Lin Ding1,2,*, Zizhen Yao1, Karla E. Hirokawa1, Thuc Nghi Nguyen1, Lucas T. Graybuck1, Olivia 5 Fong1, Phillip Bohn1, Kiet Ngo1, Kimberly A. Smith1, Christof Koch1, John W. Phillips1, Ed S. Lein1, 6 Julie A. Harris1, Bosiljka Tasic1, Hongkui Zeng1 7 8 1Allen Institute for Brain Science, Seattle, WA 98109, USA 9 10 2Lead Contact 11 12 *Correspondence: [email protected] (SLD) 13 14 15 Highlights 16 17 1. 27 transcriptomic cell types identified in and spatially registered to “subicular” regions. 18 2. Anatomic borders of “subicular” regions reliably determined along dorsal-ventral axis. 19 3. Distinct cell types and circuits of full-length subiculum (Sub) and prosubiculum (PS). 20 4. Brain-wide cell-type specific projections of Sub and PS revealed with specific Cre-lines. 21 22 23 In Brief 24 25 Ding et al. show that mouse subiculum and prosubiculum are two distinct regions with differential 26 transcriptomic cell types, subtypes, neural circuits and functional correlation. The former has obvious 27 topographic projections to its main targets while the latter exhibits widespread projections to many 28 subcortical regions associated with reward, emotion, stress and motivation. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Hippocampal Subfield Volumes Are Uniquely Affected in PTSD and Depression
bioRxiv preprint doi: https://doi.org/10.1101/739094; this version posted August 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Hippocampal subfields in PTSD and depression Hippocampal subfield volumes are uniquely affected in PTSD and depression: International analysis of 31 cohorts from the PGC-ENIGMA PTSD Working Group Lauren E. Salminen1, Philipp G. Sämann2, Yuanchao Zheng3, Emily L. Dennis1,4–6, Emily K. Clarke-Rubright7,8, Neda Jahanshad1, Juan E. Iglesias9–11, Christopher D. Whelan12,13, Steven E. Bruce14, Jasmeet P. Hayes15, Soraya Seedat16, Christopher L. Averill17, Lee A. Baugh18–20, Jessica Bomyea21,22, Joanna Bright1, Chanellé J. Buckle16, Kyle Choi23,24, Nicholas D. Davenport25,26, Richard J. Davidson27–29, Maria Densmore30,31, Seth G. Disner25,26, Stefan du Plessis16, Jeremy A. Elman22,32, Negar Fani33, Gina L. Forster18,19,34, Carol E. Franz22,32, Jessie L. Frijling35, Atilla Gonenc36–38, Staci A. Gruber36–38, Daniel W. Grupe27, Jeffrey P. Guenette39, Courtney C. Haswell7,8, David Hofmann40, Michael Hollifield41, Babok Hosseini42, Anna R. Hudson43, Jonathan Ipser44, Tanja Jovanovic45, Amy Kennedy-Krage42, Mitzy Kennis46,47, Anthony King48, Philipp Kinzel49, Saskia B. J. Koch35,50, Inga Koerte4, Sheri M. Koopowitz44, Mayuresh S. Korgaonkar51, William S. Kremen21,22,32, John Krystal17,52, Lauren A. M. Lebois37,53, Ifat Levy17,54, Michael J. Lyons55, Vincent A. Magnotta56, Antje Manthey57, Soichiro Nakahara58,59, Laura Nawijn35,60, Richard W. J. -

Slicer 3 Tutorial the SPL-PNL Brain Atlas
Slicer 3 Tutorial The SPL-PNL Brain Atlas Ion-Florin Talos, M.D. Surgical Planning Laboratory Brigham and Women’s Hospital -1- http://www.slicer.org Acknowledgments NIH P41RR013218 (Neuroimage Analysis Center) NIH U54EB005149 (NA-MIC) Surgical Planning Laboratory Brigham and Women’s Hospital -2- http://www.slicer.org Disclaimer It is the responsibility of the user of 3DSlicer to comply with both the terms of the license and with the applicable laws, regulations and rules. Surgical Planning Laboratory Brigham and Women’s Hospital -3- http://www.slicer.org Material • Slicer 3 http://www.slicer.org/pages/Special:Slicer_Downloads/Release Atlas data set http://wiki.na-mic.org/Wiki/index.php/Slicer:Workshops:User_Training_101 • MRI • Labels • 3D-models Surgical Planning Laboratory Brigham and Women’s Hospital -4- http://www.slicer.org Learning Objectives • Loading the atlas data • Creating and displaying customized 3D-views of neuroanatomy Surgical Planning Laboratory Brigham and Women’s Hospital -5- http://www.slicer.org Prerequisites • Slicer Training Slicer 3 Training 1: Loading and Viewing Data http://www.na-mic.org/Wiki/index.php/Slicer:Workshops:User_Training_101 Surgical Planning Laboratory Brigham and Women’s Hospital -6- http://www.slicer.org Overview • Part 1: Loading the Brain Atlas Data • Part 2: Creating and Displaying Customized 3D views of neuroanatomy Surgical Planning Laboratory Brigham and Women’s Hospital -7- http://www.slicer.org Loading the Brain Atlas Data Slicer can load: • Anatomic grayscale data (CT, MRI) ……… …………………………………. -

Cerebellar Granule Cells in Culture
Proc. Nati. Acad. Sci. USA Vol. 83, pp. 4957-4961, July 1986 Neurobiology Cerebellar granule cells in culture: Monosynaptic connections with Purkinje cells and ionic currents (excitatory postsynaptic potential/patch-clamp) ToMoo HIRANO, YOSHIHIRo KUBO, AND MICHAEL M. WU Department of Neurobiology, Institute of Brain Research, School of Medicine, University of Tokyo, Tokyo, Japan Communicated by S. Hagiwara, March 6, 1986 ABSTRACT Electrophysiological properties of cerebellar tissue was dissociated by triturating with a fire-polished granule cells and synapses between granule and Purkinje cells Pasteur pipette in Ca-free Hanks' balanced salt solution were studied in dissociated cultures. Electrophysiological prop- containing 0.05% DNase and 12 mM MgSO4. The cells were erties of neurons and synapses in the mammalian central centrifuged at 150 x g at 40C and the pelleted cells were nervous system are best studied in dissociated cell cultures resuspended at a concentration of about 106 cells per ml in a because of good target cell visibility, control over the contents defined medium (9). One milliliter ofthe cell suspension from of the extracellular solution, and the feasibility of whole-cell newborn rats was plated first in a Petri dish (3.5 cm in patch electrode recording, which has been a powerful tech- diameter) containing several pieces ofheat-sterilized, poly(L- nique in analyzing biophysical properties of ionic channels in lysine)-coated coverslips, and then 1 ml of fetal cell suspen- small cells. We have applied this whole-cell recording technique sion was added. One-half of the culture medium was ex- to cultured cerebellar granule cells whose electrophysiological changed with fresh medium once a week. -
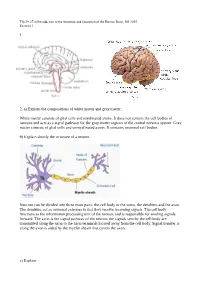
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

NEUROGENESIS in the ADULT BRAIN: New Strategies for Central Nervous System Diseases
7 Jan 2004 14:25 AR AR204-PA44-17.tex AR204-PA44-17.sgm LaTeX2e(2002/01/18) P1: GCE 10.1146/annurev.pharmtox.44.101802.121631 Annu. Rev. Pharmacol. Toxicol. 2004. 44:399–421 doi: 10.1146/annurev.pharmtox.44.101802.121631 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on August 28, 2003 NEUROGENESIS IN THE ADULT BRAIN: New Strategies for Central Nervous System Diseases ,1 ,2 D. Chichung Lie, Hongjun Song, Sophia A. Colamarino,1 Guo-li Ming,2 and Fred H. Gage1 1Laboratory of Genetics, The Salk Institute, La Jolla, California 92037; email: [email protected], [email protected], [email protected] 2Institute for Cell Engineering, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287; email: [email protected], [email protected] Key Words adult neural stem cells, regeneration, recruitment, cell replacement, therapy ■ Abstract New cells are continuously generated from immature proliferating cells throughout adulthood in many organs, thereby contributing to the integrity of the tissue under physiological conditions and to repair following injury. In contrast, repair mechanisms in the adult central nervous system (CNS) have long been thought to be very limited. However, recent findings have clearly demonstrated that in restricted areas of the mammalian brain, new functional neurons are constantly generated from neural stem cells throughout life. Moreover, stem cells with the potential to give rise to new neurons reside in many different regions of the adult CNS. These findings raise the possibility that endogenous neural stem cells can be mobilized to replace dying neurons in neurodegenerative diseases. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body.